With ageing of the general population and broadening indications, the number of pacemaker recipients is steadily increasing. The incidence of cardiac implantable electronic device (CIED) infection, a dreaded major complication, is also rising.1 The diagnosis is based on the presence of an abscessed pocket, cutaneous breakthrough of the pulse generator or vegetations attached to the pacemaker lead.2–4 On the other hand, a number of patients present with less specific clinical manifestations and a pacemaker recipient may be recurrently hospitalised for an infectious disorder of unknown origin despite detailed investigations. In the absence of proof of lead infection, further clinical management is challenging and removal of the system without confirmation of its infection is usually proposed, despite the known morbidity and mortality risks associated with the extraction procedure (0.5–2.0 %).5,6 An additional diagnostic tool to identify the presence of pacemaker system infections is therefore much needed to help solve this diagnostic problem.
In recent years, 18F-fluorodeoxyglucose positron emission tomography/ computerised tomography (18F-FDG-PET/CT) scanning has made promising contributions in a number of therapeutic areas, including imaging to detect infection in a number of organs.7 Case reports and preliminary studies suggest that 18F-FDG-PET/CT scanning may be useful in the diagnosis of pacemaker infection.8–14 Absence of hyperfixation of the lead, identified by 18F-FDG-PET/CT scanning may be an accurate sign of absence of pacing system infection. However, the limited sample sizes of previous studies do not allow the formulation of definitive conclusions.
The Diagnostic Accuracy of 18FDG-PET-CT for Pacing or Defibrillation Lead Infection (ENDOTEP) study has been designed with a sample size large enough to assess the diagnostic accuracy of 18F-FDG-PET/CT scanning in patients with suspicion of lead infection and to validate a new management algorithm in the diagnosis of lead endocarditis that includes 18F-FDG-PET/CT scanning. Here, we present the study design of the ENDOTEP study.
Methods
Study Design
This multicentre, prospective, cross-sectional study has been registered at www.clinicaltrials.gov (registration number NCT02251262) and will include 250 consecutive patients with suspected lead endocarditis over a 24-month period in six French regional centres for the investigation and treatment of CIED infection. We will include patients: 1) with clinical infection signs localised to the box (possibility of absence of lead endocarditis with negative lead culture) and; 2) patients referred for lead infection (high probability of positive lead culture). All patients satisfying the eligibility criteria will be included prospectively and consecutively in order to minimise selection bias. A whole-body 18F-FDG-PET/CT scanning (index test) will be performed in patients with suspected pacemaker/defibrillator infection 48 hours before extraction of the material and bacteriological culture (reference standard, blind to 18F-FDG-PET/CT results).
Inclusion/Exclusion Criteria
Inclusion Criteria
Patients will need to fulfill the following criteria for enrolment:
- aged >18 years;
- referred for extraction of the material (box and leads) in the context of suspicion of material infection;
- able to undergo 18F-FDG-PET/CT scanning ≤48 hours before extraction;
- provide written informed consent; and
- affiliated with a social security system.
Exclusion Criteria
Patients who meet any of the following criteria will not be included:
- pregnant or breastfeeding women;
- recent pacemaker implantation (<2 months);
- placement under judicial protection;
- participation in another study that includes an exclusion period ongoing at the time of screening; and
- unable to provide informed consent
Study Objective
Primary Objective
The primary objective of the study is to assess the diagnostic accuracy of 18F-FDG-PET/CT scanning in patients with suspected lead endocarditis.
Secondary Objectives
The secondary objectives of the study are to:
- quantify the uptake along a lead in patients with and without lead infection;
- assess the inter-observer variability in the interpretation of 18F-FDG-PET/CT scanning in this clinical situation;
- assess the impact of a previous antimicrobial treatment on the interpretation and the diagnostic accuracy of 18F-FDG-PET/CT scanning in this clinical situation;
- assess the use of whole-body 18F-FDG-PET/CT scanning for assessment of septic emboli associated with lead endocarditis; and
- assess the role of 18F-FDG-PET/CT scanning in providing differential diagnoses.
Study Protocol
The planned data collection schedule is summarised in Table 1. At the time of patient enrolment, inclusion and exclusion criteria will be checked. The following baseline characteristics will be reported: clinical examination (local signs, fever), routine blood tests with inflammatory markers, blood cultures, transthoracic and trans-oesophageal echocardiography, and presence and duration of the antibiotic therapy.
18F-Fluorodeoxyglucose Positron Emission Tomography/ Computerised Tomography Scanning
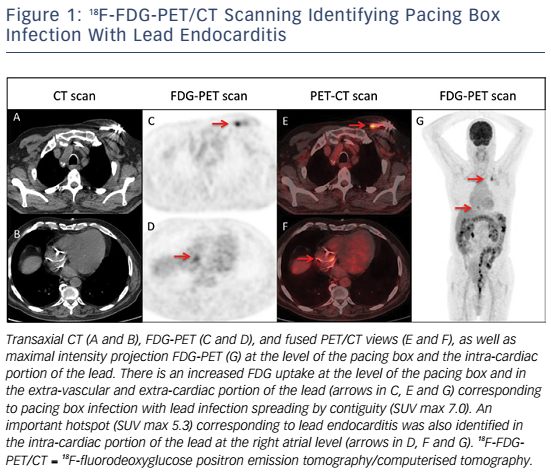
Whole-body 18FDG-PET/CT scanning will be performed within 48 hours of extraction of the entire device, after injection of 2.5–3.0 MBq/kg of 18F-FDG intravenously following a 12-hour fasting period. Patients will have a specific high-fat and low-carbohydrate diet the day before. PET/CT imaging will be performed 1 hour after 18F-FDG injection. A low-dose CT will be obtained for attenuation correction and anatomic localisation, without the use of contrast enhancement. The acquisition parameters will be: 140 kV, 80 mAs, slice thickness of 3.75 mm, pitch 1 and reconstruction interval of 1.75 mm. PET data will be acquired in 3D mode at 2 min per step. Glucose levels will be <9 mmol/l. An experienced reporter, blinded to the clinical data, will analyse the 18F-FDG-PET/CT images. The analysis will begin with visualisation of maximum intensity projection to look for pathological foci. Corrected attenuation images will be used to locate the position of the hypermetabolic foci. Then, the diagnosis of lead endocarditis will be made on non-corrected attenuation images (see Figure 1).15 This approach will also allow for assessment of septic emboli (see Figures 2 and 3). A blinded nuclear medicine physician will interpret the 18F-FDG-PET/CT scan. All 18F-FDG-PET/CT recordings will be stored until interpretation, and not used for the clinical management of the patient. Moreover, all 18F-FDG-PET/CT recordings will be sent to the coordinating centre (Bordeaux centre) to centralise interpretation blinded to the preceding one. Index test interpretations will be blinded to any other information on the patient (clinical, biology, etc).
Pacemaker Extraction
All extraction procedures will be performed in a surgical theatre by a cardiologist with the patient under general anaesthesia. When possible, extraction will be performed initially from a superior approach using a combination of simple traction or laser sheath (CVX-300® Excimer laser system; Spectranetics) as required. Where the superior approach is unsuccessful/not possible, extraction will be performed from a femoral approach using a dedicated femoral workstation (Byrd Workstation™, Cook Vascular) using a needle’s eye or gooseneck snare as appropriate.5 The pacing box and the leads will be sent separately for microbial culture.
Microbial Culture of the Extracted Material
Lead culture will be performed after the extraction procedure for all samples4,16 onto aerobic horse, chocolate PolyViteX and anaerobic sheep blood agars. The three types of agar plates will be incubated at 37°C for 10 days in aerobic, 5 % CO2 and anaerobic atmospheres, respectively. A brain heart infusion broth supplemented with meat liver agar and IsoVitaleX™ (BD Diagnostic Systems) will be also inoculated and will be subcultured for 10 days on PolyViteX™ (bioMérieux) chocolate agar plates in a 5 % CO2 atmosphere if cloudy (or systematically at 1 month). Strain identification will be performed using by mass spectrometry, using matrix-assisted laser desorption/ ionisation. Antibiotic susceptibility testing (AST) will be performed for all detected strains, using the disk diffusion method.
The diagnosis of lead endocarditis will be confirmed in the following cases:
- lead culture growing the same microorganism as one or more blood cultures;
- lead culture growing the same organism as the pulse generator culture; or
- cultures from at least two leads growing the same organism.
We will consider the bacterial strains to be the same when the identification and the AST results are identical for both samples.
Sample Size Calculation and Statistical Analysis
A negative bacteriological lead culture result may be found in 10–25 % of cases,16 even in absence of previous antimicrobial therapy prior to the extraction procedure. The first hypothesis of the ENDOTEP study is that a new strategy adding 18F-FDG-PET/CT scanning to the current diagnosis strategy applied before extraction may avoid or reduce these false-positive results. To evaluate the potential benefits and risks of this new strategy, we will need a randomised trial comparing it with the standard strategy. However, the decision to carry out such a trial has to be justified by valid data on the diagnostic accuracy of the 18F-FDG-PET/CT scanning, attesting of a high sensitivity of this test. High sensitivity would maximise the negative predictive value, which is needed to validate the safety of not extracting a pacemaker in patients with a negative 18F-FDG-PET/CT scan result. In addition, high sensitivity would enable the diagnosis of lead endocarditis in patients with fever of unknown origin. Thus, the primary objective of this study is the estimation of the sensitivity.17,18The main analysis will estimate the 95 % confidence interval of PET/CT sensitivity, and compare its lower bound with the predefined value of 90 %. If 250 patients are included, and assuming a prevalence of at least 75 % of cases with positive bacteriological cultures, this comparison will have at least a 90 % power with a type I error of 5 % if the observed sensitivity is ≥96 %. In that case, if the specificity is >90 %, the negative predictive value of PET/CT will be between 71 and 88 % when the prevalence of infection is between 75 and 90 %. Possible variations of the diagnostic accuracy of 18F-FDG-PET/CT scanning according to previous antimicrobial therapy and pocket infection will be analysed with an adjusted logistic model if necessary.
Inter-observer agreement of visual interpretation of 18F-FDG-PET/CT scanning will be estimated using the Cohen’s kappa coefficient.
Discussion
18F-FDG is a nonspecific tracer of increased glucose metabolism that has a high sensitivity for the detection of malignant cells. In recent years, 18F-FDG-PET/CT scanning has made promising contributions in different areas including imaging to detect infection at different organ sites. In this study, we will evaluate the accuracy of the 18F-FDG-PET/CT scanning for lead endocarditis diagnosis, by performing this exam in all consecutive patients referred for pacemaker extraction.
The high number of planned patients (250) will allow for the assessment of:
- inter-observer variability in the interpretation of 18F-FDG-PET/CT scanning in this clinical situation – this point is the first step before integrating this tool into a new diagnostic algorithm;
- the diagnostic accuracy of 18F-FDG-PET/CT scanning in patients with a suspicion of infection of pacemaker or defibrillator leads – we will include a large number of patients with expected positive (lead endocarditis) and negative (infection restricted to the box) culture of the leads and, therefore, it will be possible to evaluate sensitivity, specificity and predictive values;
- the impact of a previous antimicrobial treatment in the interpretation of 18F-FDG-PET/CT scanning in this clinical situation – including patients who have and have not received antibiotics will help clarify the impact of previous antimicrobial treatment on the accuracy of 18F-FDG-PET/CT scanning for lead endocarditis diagnosis; and
- the role of whole-body 18F-FDG-PET/CT scanning in diagnosing other areas of infection.
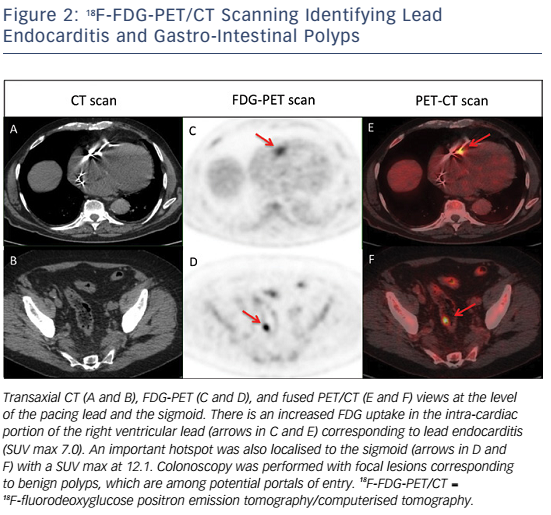
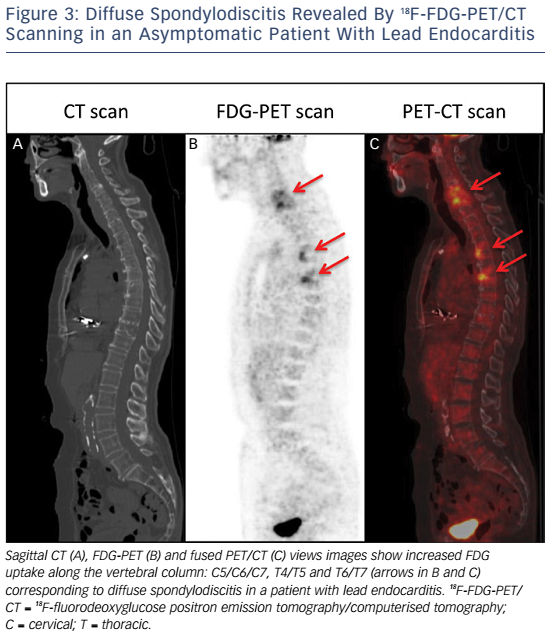
High mortality rates associated with lead endocarditis is partly due to the presence of septic embolisms; septic pulmonary embolisms and spondylodiscitis seem to be the most frequent types.19–21 The diagnosis of septic embolisms in patients with lead endocarditis can be challenging. Indeed, magnetic resonance imaging cannot be performed in the vast majority of patients with a cardiac implantable device. The risk of septic embolism associated with lead endocarditis is therefore probably under-estimated as septic embolisms are not systematically sought in many cases despite possible important clinical implications (adaptation in terms of type, administration mode and duration of antibiotic therapy).22,23
This prospective study may help position 18F-FDG-PET/CT scanning in the diagnostic algorithm for lead endocarditis. It is not a substitute for trans-oesophageal echocardiography, which contributes different information; for example, the size of vegetations is an important factor when planning the lead extraction procedure.5 On the other hand, trans-oesophageal echocardiography provides information restricted to the intra-cardiac portion of the leads and requires the presence of visible vegetations. In contrast, 18F-FDG-PET/CT scanning provides information on the intra- and extra-cardiac portions of the leads and may be highly beneficial when vegetations are not visible. This tool may prove to be effective in diagnosing septic emboli and therefore may be proposed in all the patients referred for device extraction as the demonstration of the presence of asymptomatic spondylodiscitis, for example, may have direct therapeutic implications. 18F-FDGPET/CT scanning may also be proposed in pacemaker recipients hospitalised for an infectious disorder of unknown origin despite detailed investigations including trans-oesophageal echocardiography. If the 18F-FDG-PET/CT scan is positive (hyperfixation on a lead) then the pacing system should be extracted. If the 18F-FDG-PET/CT scan is negative (no hyperfixation on a lead) then the pacing system may be left in situ with close clinical monitoring and follow-up.
The potential disadvantages of this diagnostic tool include its limited availability and relatively high cost; 18F-FDG-PET/CT scans performed in France costs approximately 589 euros. However, it may prove to be costeffective given that its used could avoid unnecessary implant extractions.
Conclusion
The ENDOTEP study will examine the potential use of 18F-FDG-PET/CT scanning as a diagnostic tool for lead infection diagnosis in patients with fever of unknown origin. Here, we have presented the design of this prospective, multicentre trial.