Myocarditis is difficult to diagnose due to its heterogeneous clinical presentation. Patients often present with nonspecific symptoms and there is no easy-to-use and widely available diagnostic test. Although the clinical manifestations of myocarditis are heterogeneous, up to one-third of cases progress toward dilated cardiomyopathy (DCM).1,2 Myocarditis is a major cause of DCM, which is a significant cause of heart failure and sudden cardiac death in young adults.3 DCM due to myocarditis is the major underlying reason for cardiac transplantation. Patients with acute myocarditis often present with acute coronary syndrome-like symptoms;3,4 those with chronic myocarditis are likely to have symptoms of DCM and heart failure.
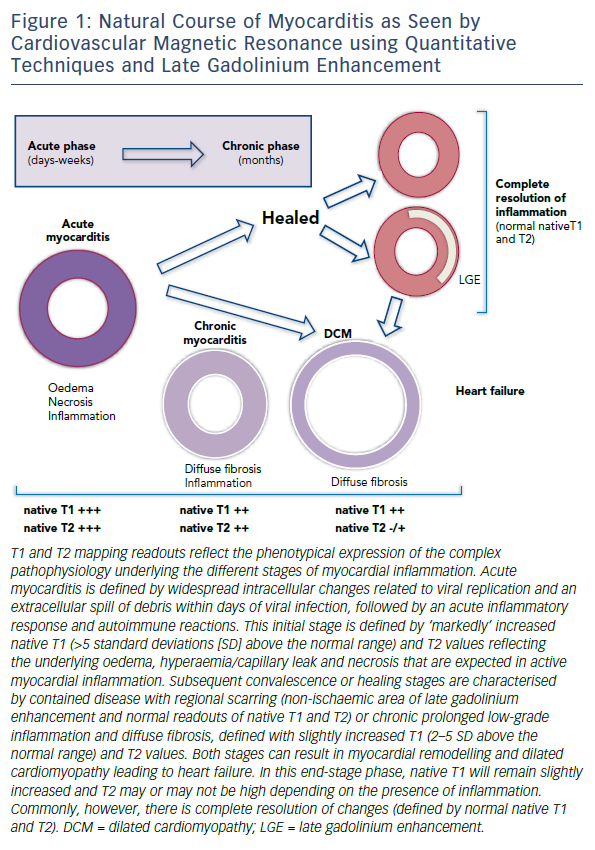
Myocarditis due to viral infection is the most common and best understood clinical presentation of inflammatory cardiomyopathy;5,6 however, other triggers, including systemic autoimmune inflammation and cardiac toxins such as alcohol and chemotherapy, are also increasingly recognised causes of myocarditis.7,8 Irrespective of the original trigger, the pathophysiology of myocardial injury in all forms of myocarditis is underlined by autoimmune myocardial inflammation that progresses through several disease stages. After the initial trigger, acute myocarditis is characterised by inflammation, oedema and necrosis due to autoimmune responses to cardiac antigens, leading to regional myocardial injury and scarring.8,9 The chronic stage features diverse phenotypes, including healed myocardium (with possible residual scarring) or latent low-grade autoimmune myocardial inflammation, leading to remodelling, DCM and heart failure (Figure 1).10,11
There are several challenges in the clinical management of myocarditis.12 There is no specific treatment available for myocarditis or its sequelae. Studies using anti-inflammatory treatment in haemodynamically-stable patients have not shown any benefit, partly because they have recruited chronic myocarditis patients in the advanced stages of DCM and heart failure.7 The broad range of clinical manifestations may also present a challenge. Despite significant research, little is known about the mechanisms involved in post-inflammatory cardiac remodelling leading to DCM. Alterations in the myocardial extracellular matrix by matrix metalloproteinases and tissue inhibitors of metalloproteinases are critical during myocardial fibrosis and cardiac remodelling; however, the pathways that lead to progression to DCM are unclear. Studies on experimental models of myocarditis have led to the discovery that the autoimmune inflammatory process plays a decisive role in acute inflammatory injury and progression to chronic inflammation and remodelling.6,8,13 The interplay between the pro- and anti-inflammatory cytokine pathways seems to determine the course of disease, and in predisposed individuals leads to the development of DCM.13–17 The role of interleukin-17 in moderating the progression from acute myocarditis to chronic inflammation and remodelling has been highlighted in several studies.10,14,18 In animal models, short-term use of anti-interleukin-17 has ameliorated the disease, identifying a potential treatment target.19–21
In line with European Society of Cardiology clinical practice guidelines, the diagnosis of myocarditis relies on confirmation by endomyocardial biopsy.8 This is an invasive, complex procedure with attendant risks and variable diagnostic yield. With expertise largely limited to the tertiary centres, the use of endomyocardial biopsy in clinical practice varies; in most centres it is reserved for patients with severe presentation and an aggressive disease course, where histological confirmation of giant cell myocarditis offers the hope of treatment with steroids. In addition to the issues already outlined, its limited sensitivity as a result of sampling-error and inconsistencies regarding the histological and immunohistochemical criteria for diagnosis have restricted its use.22
The lack of a simple, reliable non-invasive test leaves a considerable proportion of patients undiagnosed and has contributed to the difficulty in finding new treatments as patients often present with advanced disease that is harder to manage. Cardiovascular magnetic resonance (CMR) imaging is gaining recognition over and above the other non-invasive imaging techniques in the diagnosis of clinicallystable patients.23,24 In addition to its more accurate measurement of biventricular volumes and systolic function, CMR imaging is capable of giving clues as to the presence and distribution of inflammatory damage in the myocardium through its tissue characterisation capabilities.
CMR imaging based on the Lake Louise criteria (LLC) represented the first steppingstone to a non-invasive diagnostic option.25 Previous studies had found a variable correlation between clinical and histological evidence of myocarditis and abnormal findings in CMR imaging when focusing on the three main hallmarks of myocardial inflammation: oedema, hyperaemia/capillary leak and necrosis or fibrosis. CMR-LLC is based on a comprehensive protocol that includes T2-weighted, T1-weighted (before and immediately after contrast administration) and late gadolinium enhancement (LGE) imaging sequences. Positive findings include the identification of oedema on T2-weighted images (oedema ratio >1.9 or qualitative presence of an area of regional high signal intensity), hyperaemia and capillary leakage on T1-weighted images (increased global myocardial early gadolinium enhancement ratio between the myocardium and skeletal muscle; relative enhancement ratio >4), and at least one focal lesion with non-ischaemic regional distribution in sequence. Several LGE patterns are characteristic in patients with myocarditis. Areas of focal LGE are typically localised to the sub-epicardial regions of the left ventricle and extend to a variable extent through the ventricular wall. LGE may, however, appear patchy or diffuse. The sub-endocardium is not usually involved, allowing differentiation from ischaemic injury. Any LGE/T2 pattern or finding can definitively suggest the different aetiology of myocardial inflammation. Although additional findings – such as pericardial effusion, global or regional left ventricular systolic function, or transient increase in wall thickness – may support the diagnosis of active myocarditis, they are not included in the proposed diagnostic criteria. In the current guidelines, CMR supports endomyocardial biopsy, by informing on the likelihood of myocarditis prior to the procedure.8
The strength of LLC is in allowing confirmation of disease when the imaging findings are present, leading to improved insights of disease in terms of its natural history and sequelae. It supports the diagnosis in a significant percentage of patients; however it lacks sufficient sensitivity, i.e. it has poor ability to exclude disease, despite giving a convincing clinical picture. This is due to the limitations of the criteria used to detect the more diffuse inflammatory involvement of acute myocarditis. The predominant diffuse myocardial inflammation and intracellular and interstitial oedema7 leads to insufficient regional redistribution of gadolinium, which results in a normal LGE image.26 Abundant interstitial oedema and faster water exchange with cells with dysfunctional membranes lead to magnetisation transfer from the extracellular gadolinium to the intracellular water, further reducing the contrast.27 Similarly, appreciation of the increased T2 signal intensity relies on regional differences. The oedema ratio is based on the relative comparison of signal to skeletal muscle. This can be also affected, thus resulting in a pseudo-normalised value.28
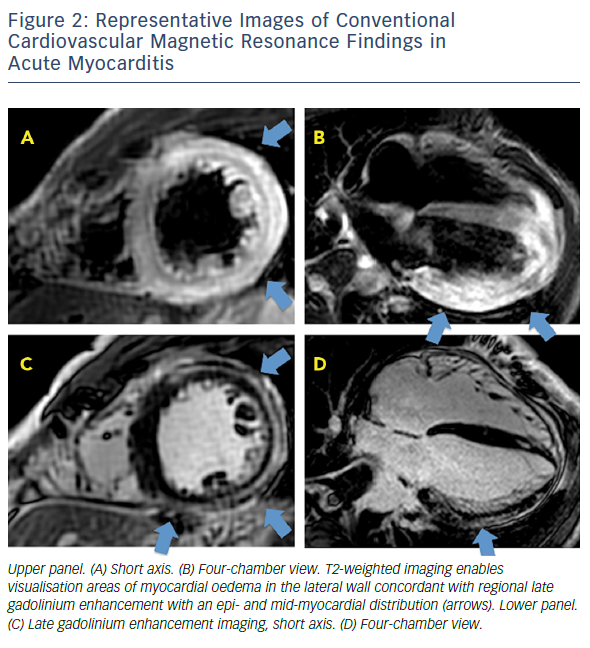
Relative enhancement is technically limited by low reproducibility and susceptibility to artefacts. The contribution of relative enhancement to diagnostic accuracy was based on two relatively small studies27–30 and was subsequently shown to be of little relevance; a prospective study applying LLC with T2 and/or LGE resulted in higher diagnostic accuracy and negative predictive value in comparison to any two out of three approach.31 As such, the Society for Cardiovascular Magnetic Resonance recommendations classify the relative enhancement ratio as ‘optional’ in the myocarditis imaging protocol.32
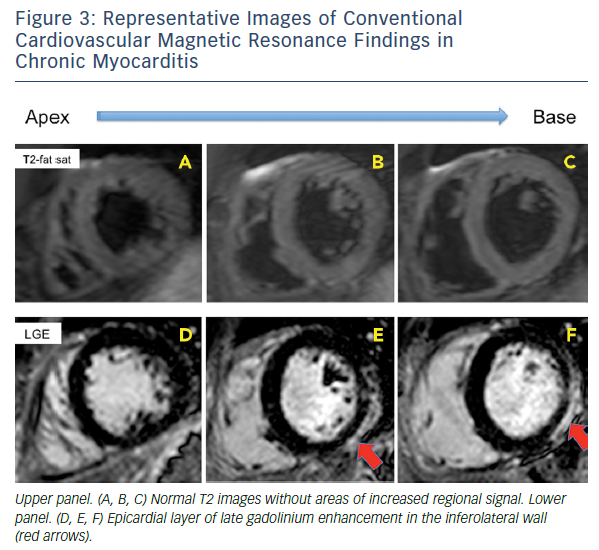
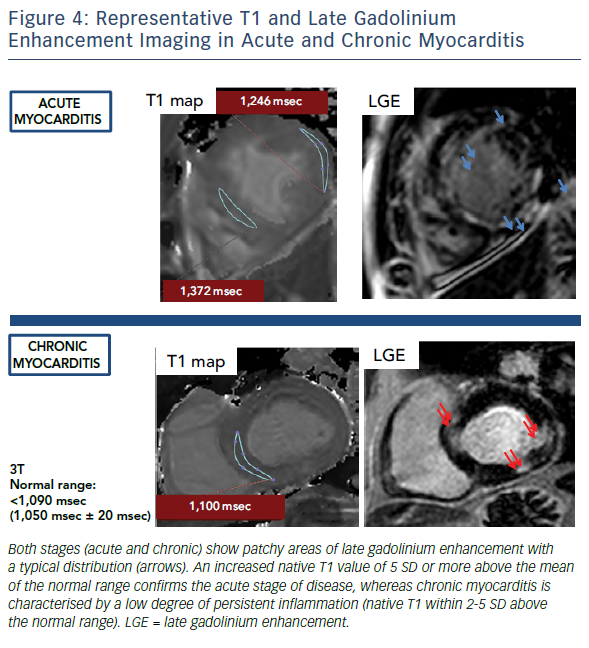
When CMR imaging is performed in patients with chronic myocarditis or symptoms of heart failure the diagnostic challenge is even harder: the predominant signs (if present) are the functional impairment and non-ischaemic LGE patterns (midwall stria), which may more readily underpin the diagnosis of idiopathic DCM.33–35 Persistent low-grade interstitial myocardial inflammation is not visualised with T2-imaging or picked up by LGE, which will only reveal the presence of residual and regional cell death. These patients frequently remain unidentified and are classified as having non-specific nonischaemic DCM.34,36,37
Novel CMR Techniques
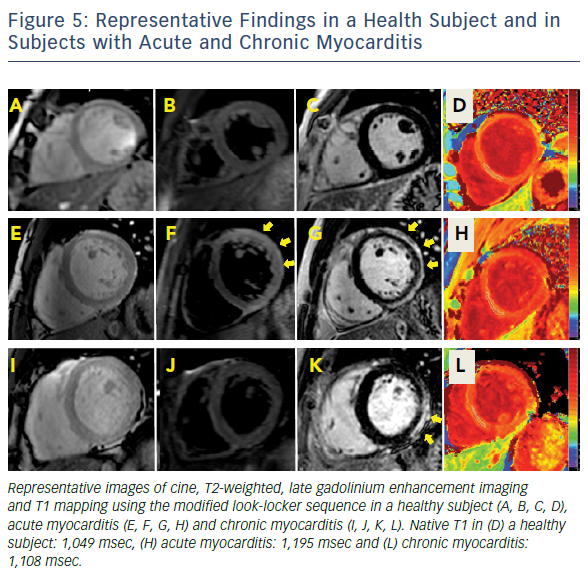
In the past few years, novel quantitative techniques have been developed and have evolved rapidly, leading to the expectation that they will improve the understanding of non-ischaemic cardiomyopathies. Parametric maps encoding quantitative measurement of magnetic T1 and T2 relaxation times have enabled more detailed tissue characterisation, offering the possibility of overcoming some of the limitations of LLC in the diagnosis of myocarditis. Both parameters have shown superior diagnostic performance compared to LLC, significantly adding to the ability of CMR to confirm or exclude the presence of myocardial inflammation.37–39 T1 and T2 mapping has outperformed relative enhancement and T2-weighted imaging in clinical studies with active myocarditis. Evidence supports the use of native T1 and T2 measurements to detect myocardial inflammation over and above T2-weighted imaging, given its higher sensitivity to detect water.38,39 Native T1 and T2 reflect the intracellular and diffuse interstitial components of inflammation. Furthermore, as the inflammatory response (i.e. oedema and hyperaemia) is expected to regress after the early acute stage, native T1 shows a progressive reduction over time.38 The readouts of both parameters have the potential to approximate pathophysiological complexity during the different stages and grades of acute myocarditis. Convalescent stages of the disease can be defined by quiescent readouts (native T1 within normal range, absence of LGE, areas of resolved injury by LGE), or a low-grade persistent inflammation (native T1 within 2-5 standard deviations above the normal range, presence or absence of LGE) (Figure 1). Native T2 mapping has improved our understanding of the underlying pathological processes in the different stages of myocardial inflammation and deciphered the nature of the signal captured with T1 mapping, particularly in the chronic stages where native T1 also reacts to diffuse fibrosis.37,40 Native T2 has higher diagnostic accuracy than LLC in the detection of myocarditis in patients with chronic myocarditis and recent-onset heart failure and reduced left ventricular function.33
A recent study investigated concordance between quantitative techniques and histological readouts in myocarditis, showing a good concordance between T1 and T2 mapping and histological evidence of myocardial inflammation. Furthermore, whereas native T1 was able to detect all patients with abnormal myocardium, native T2 identified those subjects with chronic myocarditis where inflammation remained active.35 Native T1 detected all patients, including those with DCM, related to their functional impairment41–43, as well as inform on their significantly worse prognosis, better than late gadolinium enhancement and ejection fraction.43
As novel diagnostic techniques, mapping methods remain site- and vendor-specific, and the accuracy and precision of T1 measurements may vary between CMR systems, sequences and post-processing approaches.44 Local normal ranges and abnormal cut-off values need to be determined in each particular centre before such methods are included in clinical protocols. The use of different T1 and T2 methods can explain thus some of the disparities between the findings of different studies. Histological correlation is clearly informative, as it reveals the difficulty of clinically diagnosing myocarditis in practice. Endomyocardial biopsies are often performed late in the course of disease; whereas they can inform on the presence of myocardial inflammation based on the cellular infiltrate, they cannot evaluate the extent of myocardial oedema, an important disease marker. T1 and T2 mapping indices, in contrast, strongly relate to water signals, clarifying the histological blind spots in inflammatory myocardial disease.
Future Molecular Imaging Research
One potential focus for research in the field is the development of molecular imaging. Few studies in animal models have explored the potential of molecular imaging to detect or monitor the effectors and/or modulators of inflammation based on the ability of radiolabeled ligands to measure pathophysiological processes. Although nonspecific, 18F-fluorodeoxyglucose positron emission tomography is the most frequently used radioligand for non-invasive detection of inflammation.8,45 Radioligands that bind to translocator protein 18 kDa, which is a well-established biomarker for brain injury and inflammation, are also being developed for the early detection of myocarditis. Besides this, the detection of immune cell infiltration in a murine model of autoimmune myocarditis by 19F-flurorine CMR in vivo has been explored with the use of biochemically-inert perfluorocarbons. These perfluorocarbons are taken up by circulating monocytes/ macrophages after intravenous injection and are specifically recruited to areas of inflamed myocardium, which can be seen with CMR.46
An alternative potential focus for research is the development of molecular labels for CMR-based virus detection.47,48 The development of CMR agents directed against antigens in the viral capsid might enable the non-invasive detection of myocardial inflammation. Furthermore, the potential contribution of positron emission tomographic cardiac imaging for diagnosing inflammatory cardiomyopathies has been explored in cardiac sarcoidosis.49–51 Its role in the setting of viral myocarditis has yet to be investigated.
Conclusion
Among the imaging techniques available, CMR is an important noninvasive diagnostic tool in patients with suspected myocarditis. Tissue characterisation capabilities are continuing to evolve, enabling CMR to offer a stronger and increasingly reliable alternative to invasive biopsy in clinically-stable patients. Longitudinal studies with non-invasive imaging techniques, which are risk- and radiation-free, will improve the overall understanding of the natural course of myocarditis. Future research will decipher whether a complementary approach with T1 and T2 mapping may lead to an updated CMR algorithm in practice.







