The use of dual antiplatelet therapy (DAPT) after stent implantation in a percutaneous coronary intervention (PCI) is the standard treatment. The first randomised controlled trial (RCT) to establish the superiority of DAPT versus oral anticoagulant treatment among patients undergoing PCI was the Intracoronary Stenting and Antithrombotic Regimen (ISAR) trial, published in 1996.1 Since then, more than 35 RCTs have been carried out, with more than 225,000 participants, to assess different aspects of DAPT in this context, including the ideal approach of antiplatelet drug and the optimal duration of treatment.
With the advent of the first bare metal stents (BMS), it was established that DAPT was needed for a month by studies such as the Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS).2 Until clopidogrel was approved by the FDA in 1997, the drug used together with acetylsalicylic acid (ASA) was ticlopidine.
The duration of treatment with DAPT was extended to 3 months after the approval of the first drug-eluting stents (DES) containing sirolimus, and to 6 months after the release of paclitaxel DES. These periods were established without any clinical evidence. The length was extended to 12 months after the findings of wide registries documenting a sustained risk of late stent thrombosis beyond 6 months.3,4 This risk was not identified by the first clinical trials for DES.5 The concern raised among the medical community regarding late and very late thrombosis events with the use of these first-generation DES created the need to assess prolonged DAPT regimens.
Subsequently, with the introduction of second and third generation DES, such as thinner struts, the -limus drugs, and more biocompatible or biodegradable polymers, which have decreased the risk of late and very late thrombosis to numbers similar or even lower than the BMS, there has been a drift in the approach to DAPT.6,7
Although DAPT continues to play a key role in reducing the risk of late and very late thrombosis, the significant related risk of bleeding implies that, currently, prolonged 12-month DAPT is not generally justified. On the other hand, there is sustained evidence that DAPT can reduce long-term cardiovascular events independently of the prevention of stent thrombosis, by preventing thrombotic events of atheromatous plaques, especially in patients who have had acute coronary syndrome (ACS).8,9
DAPT has moved from a local focus on the prevention of stent thrombosis to be considered part of a global strategy of treatment that provides the patient with overall protection against vascular thrombotic events, especially cardiac but also cerebral.
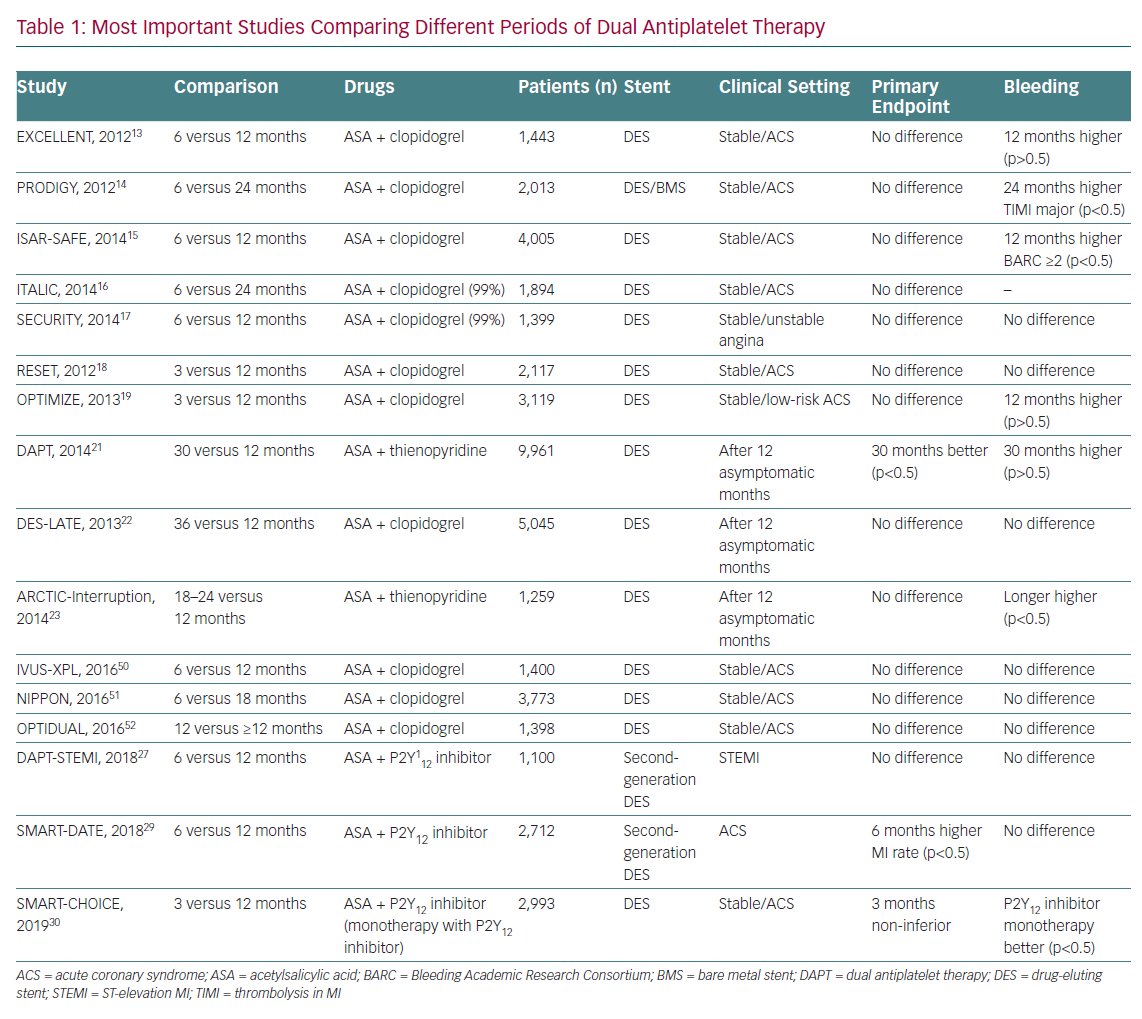
Studies carried out in recent years aimed to establish the minimum safe duration of DAPT for the new DES as well as considering the potential benefit of continuing DAPT over 12 months in certain patients. These studies are summarised in Table 1.
In 2016, the American College of Cardiology and the American Heart Association published an update of the clinical practice guidelines on the duration of DAPT in patients with coronary artery disease (CAD).10 These guidelines were developed based on the results of a systematic review of all the studies carried out on this topic.11 More recently, the European Society of Cardiology (ESC), in collaboration with the European Association of Cardiothoracic Surgery, published an update of the guidelines on DAPT in CAD.12 Both documents show a high degree of consensus, but in this article we will focus on the recommendations in the European guidelines.
Since the publication of these two practice guidelines, several studies have been published which address similar questions and many others are in the inclusion or follow-up phase and the findings will probably have an impact on future guidelines. We will, therefore, comment on some of these most interesting studies.
The clinical context in which the patient is being treated must always be considered when making a decision about the type of antiplatelet drug and the duration of treatment, so we will discuss patients undergoing percutaneous revascularisation in a stable situation separately from those revascularised in the setting of ACS. Moreover, the management of patients undergoing percutaneous revascularisation who also have a high risk of bleeding or need chronic oral anticoagulation require a separate mention.
DAPT After Percutaneous Revascularisation in Stable Coronary Artery Disease
There are no clinical trials that assess the duration of DAPT exclusively in stable patients, so all the recommendations have been drawn from subgroups from wider trials. There is also a lack of trials that evaluate the use of prasugrel or ticagrelor as an alternative to clopidogrel in the stable context, although their use is accepted in selected patients who have unsatisfactory previous use or clinical resistance to clopidogrel, drug allergy or a high risk of ischaemia.
DAPT for at Least 12 Months Versus 3–6 Months
Several trials have aimed to assess this aspect, all with very similar results, and have led to the proposed recommendations.
The Efficacy of Xience/promus versus Cypher in rEducing Late Loss after stENTing (EXCELLENT) compared a strategy of 6 months versus 12 months of DAPT (ASA and clopidogrel).13 It included 1,443 patients treated with DES. At 1 year, the 12-month group had a target vessel failure rate slightly lower than the 6-month group (4.3% versus 4.8%), at the expense of a non-significant increase in bleeding. The findings were independent from the clinical context (stable versus unstable).
The Prolonging Dual-Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) compared a strategy of 6 months versus 24 months of DAPT (ASA and clopidogrel). It included 2,013 patients randomised to four different stents (one BMS and three DES). At 2 years, no significant differences were found in the combined primary endpoint of major adverse coronary events (MACE) but there was higher significant bleeding in the 24-month group, even more so in the subgroup of patients with stable CAD.14
The Intracoronary Stenting and Antithrombotic Regimen: Safety And EFficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) compared a strategy of 6 versus 12 months of DAPT (ASA and clopidogrel).15 It included 4,005 patients treated with DES. and included both stable and unstable patients. It concluded that the 12-month strategy did not provide any benefit over the 6-month strategy. The Is There A LIfe for Drug-eluting Stents (DES) After Discontinuation of Clopidogrel (ITALIC) and Second Generation Drug-Eluting Stent Implantation Followed by Six- Versus Twelve-Month Dual Antiplatelet Therapy (SECURITY) trials had a similar design and results to ISAR-SAFE.16,17
The REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation (RESET) trial compared a strategy of 3 versus 12 months of DAPT (ASA and clopidogrel).18 It included 2,117 patients treated with zotarolimus DES. There were no significant differences in MACE between the two groups.
The Optimized Duration of Clopidogrel Therapy Following Treatment With the Zotarolimus-Eluting Stent in Real-World Clinical Practice (OPTIMIZE) trial compared a strategy of 3 versus 12 months of DAPT (ASA and clopidogrel).19 It included 3,119 patients treated with zotarolimus DES. There were no significant differences in MACE between the groups.
A meta-analysis comparing the strategy of 12 months of DAPT after the implantation of a DES versus no more than 6 months of DAPT concluded that the 1-year strategy does not provide any advantage in terms of survival, stent thrombosis or acute MI and substantially increases the risk of major bleeding.20
DAPT for 12 Months Versus Longer Treatment Strategies
The main characteristics and results of trials that address 12 months of DAPT treatment versus longer treatment strategies are summarised here.
The Dual Antiplatelet Therapy (DAPT) study compared a strategy of 12 months versus 30 months of DAPT.21 It included 9,961 patients treated with DES without ischaemic or haemorrhagic events in the first year of treatment after stent implantation. In the 30-month arm, there was a significant decrease in thrombosis (0.4% versus 1.4%, p <0.001) and major adverse cardiac and cerebrovascular events (4.3% versus 5.9%, p<0.001). This is associated with a significant increase in the risk of bleeding (2.5% versus 1.6%, p<0.001) and an almost significant increase in mortality from any cause.
Three independent meta-analyses that included the results of the Optimal Duration of Clopidogrel Therapy With DES to Reduce Late Coronary Arterial Thrombotic Event (DES-LATE; 5,045 patients) and the Assessment by a Double Randomisation of a Conventional Antiplatelet Strategy Versus a Monitoring-Guided strategy for Drug Eluting Stent Implantation and, of Treatment Interruption Versus Continuation 1 Year After Stenting-Interruption (ARCTIC-Interruption; 1,259 patients) trials reached conclusions consistent with a possible increase in global mortality with the prolongation of DAPT.22,23
These studies show that maintaining long-term DAPT in patients with stable CAD treated with DES confers a benefit in terms of secondary prevention of ischaemic events and reduction of stent thrombosis, but at the expense of an increased risk of bleeding and a potential increase in global mortality.
Taking all this into account, the class I level A recommendation is that maintenance of DAPT is not systematically recommended beyond 6 months and the duration should be individualised according to the patient’s risk profile. It will be necessary to assess the patient’s ischaemic and bleeding risk in the medium to long term, for which several tools have been developed. Those recommended in the European guidelines are the PRECISE-DAPT score and the DAPT score. The PRECISE-DAPT score decides the duration of DAPT at the time of stent implantation.24 The DAPT score can be used to make decisions to prolong DAPT after an uneventful first year post-PCI. Of note, the type of stent implanted is important when assessing the benefit of extending DAPT beyond one year. This benefit is clearer in patients with first-generation DES, although these are no longer used.
There have been no studies assessing the optimal duration of DAPT after implantation of bioabsorbable scaffolds, but there is evidence of an increased risk of stent thrombosis in the first month and in the long term, which is why, in patients treated with these stents, it seems reasonable to recommend DAPT for at least 12 months, and even prolong it when the risk of bleeding is low.
DAPT After Percutaneous Revascularisation in Acute Coronary Syndrome
The usefulness of DAPT with ASA and clopidogrel for 1 year in patients with ACS has been amply demonstrated.8,9 There are also studies that demonstrate the superiority of ticagrelor (PLATelet inhibition and patient Outcomes [PLATO] trial) and prasugrel (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction [TRITON-TIMI]) versus clopidogrel in this context.25,26 Although both prasugrel and ticagrelor significantly increase the risk of major TIMI bleeding not related to surgery, the risk–benefit ratios are favourable, with a number needed to treat to prevent a primary outcome of 46 and 53, respectively, and the number needed to harm of 167 for both drugs.
For these reasons, the recommendation is to prescribe DAPT for 1 year after ACS has been established, preferably with ticagrelor or prasugrel, unless there are contraindications (class I level C).
DAPT for 12 Months Versus 3–6 Months
Following the idea that longer DAPT exposes the patient to higher bleeding risk and therefore poorer prognosis and in line with the studies that seek to shorten the duration of DAPT in stable patients, a line of research is also arising in the setting of ACS.
The Dual Antiplatelet Therapy after drug-eluting stent implantation in ST-elevation MI (DAPT-STEMI) trial was designed to show that limiting DAPT to 6 months in patients with event-free ST-elevation MI (STEMI) results in a non-inferior clinical outcome (composite of all-cause mortality, any MI, any revascularisation, stroke and TIMI major bleeding at 18 months after randomisation) versus DAPT for 12 months.27 There were 1,100 patients enrolled. The authors concluded that the short-term strategy was non-inferior in the long term in patients with event-free STEMI at 6 months after primary PCI with second generation DES.
A later meta-analysis included 17,941 patients from three RCTs and eight RCT sub-analysis allocated to two groups according to the DAPT strategy. It concluded that a short duration of DAPT may be safely considered, with similar rates of recurrent thrombotic complications compared with the standard 12 months, and similar mortality.28
After this meta-analysis was published, new data have been released addressing this same topic. The Safety of 6-month Duration of Dual Antiplatelet Therapy After Acute Coronary Syndromes (SMART-DATE) trial aimed to prove that a 6-month duration of DAPT is non-inferior to a conventional 12-month or longer duration of DAPT at preventing the occurrence of major adverse cardiac and cerebrovascular events at 18 months after second-generation DES implantation in patients with ACS.29 A total of 2,712 patients were included and randomised. The authors found an increased risk of MI with the short-term strategy and concluded that prolonged DAPT in patients with ACS without excessive risk of bleeding should remain the standard of care.
The P2Y12 Inhibitor Monotherapy Versus Extended DAPT in Patients Treated with Bioresorbable Scaffold (SMART-CHOICE) trial compared the efficacy and safety of P2Y12 inhibitor monotherapy versus aspirin plus P2Y12 antagonist following 3-month of DAPT in patients undergoing PCI with DES. Of the 2,993 patients included, 58% had ACS. The results showed that short-duration DAPT (3 months) followed by P2Y12 inhibitor monotherapy is non-inferior to longer-duration DAPT (12 months) among unselected patients undergoing PCI with a DES.30
The results of all the above-mentioned studies were published after the ESC guidelines were published. Therefore, no specific mention on this strategy is made apart from the 6 months recommendation in patients at high risk of bleeding.
DAPT for 12 Months Versus More Than 12 Months
Patients with ACS have a high cardiovascular risk beyond the first year and intensive DAPT has been shown to be effective in reducing the rate of new recurrent ischaemic events. The risk–benefit balance is not so clear and several trials have been developed to try to clarify the issue.
The Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin (PEGASUS) study included 21,162 patients with a previous MI (between 1 and 3 years before the start of the study) and a high-risk profile. Monotherapy with ASA was compared with two DAPT regimens with ASA and ticagrelor (60 mg or 90 mg). Although the main combined result of efficacy (cardiovascular death, MI and stroke at 3 years) was significantly better in the ticagrelor groups (7.85% with 90 mg and 7.77% with 60 mg versus 9.04% in ASA monotherapy), a significant increase in TIMI bleeding was also observed (2.6% with 90 mg, 2.3% with 60 mg and 1.06% in ASA monotherapy). Therefore, it is not possible to obtain a net benefit with 90 mg ticagrelor and the benefits are marginal with 60 mg ticagrelor.31
For these reasons, the generalised use of DAPT with ASA and ticagrelor beyond a year after an ACS is discouraged and more individualised use is advocated, considering the ischaemic and haemorrhagic risks of each particular patient.
The DAPT study compared 12 months versus 30 months of DAPT (ASA and either clopidogrel or prasugrel).21 A non-pre-specified analysis of the sub-group who had an MI (3,567 patients) found a significant reduction in thrombosis in the 30-month group (0.5% versus 1.9%; p<0.001), as well as of new infarction (2.2% versus 5.2%; p<0.001), at the expense of an increase in moderate or severe bleeding, according to the Global Use of Strategies to Open Occluded Arteries (GUSTO) scale (1.9% versus 0.8%; p=0.005) and the same mortality rate for all causes.
Recently, a meta-analysis including clinical trials that assess the use of DAPT (clopidogrel, prasugrel or ticagrelor) beyond 12 months after ACS has been carried out. The conclusions are similar: reduction in cardiovascular events at the expense of an increase in bleeding with neutral or negative impact on overall mortality.32
Although these findings seem to show a consistent class effect among the three P2Y12 receptor inhibitors, the use of 60 mg ticagrelor is recommended in patients with a low risk of bleeding in whom it is decided to continue with DAPT after 1 year. This recommendation is based on the fact that ticagrelor is the most widely studied drug and has the most complete trials.
DAPT Duration in Patients with a High Risk of Bleeding
The majority of clinical trials exclude patients with a high risk of bleeding, although the definition of high bleeding risk had not been well established until the recently published Academic Research Consortium for High Bleeding Risk definitions.33 Nevertheless, the evaluation of shorter DAPT strategies allows inferring results applicable to this patient group. A meta-analysis, with data from six trials, compared the strategy of 12 months of DAPT versus 3 or 6 months in 4,758 patients after an ACS.34 Reduction of DAPT to 6 months resulted in a non-significant increase in the risk of thrombosis or MI, while reducing DAPT to 3 months significantly increased this risk.
There are some studies that have shown safety with 3 months of DAPT, but they were performed with outdated DES. However, there are ongoing studies with current DES and registries that guarantee the safety of new generation DES with 3 months of DAPT.
To date, only one trial has specifically assessed the population of patients with the highest bleeding risk, the Prospective Randomized Comparison of the BioFreedom Biolimus A9 Drug-Coated Stent versus the Gazelle Bare-Metal Stent in Patients at High Bleeding Risk (LEADERS FREE) trial.35 In this study, 2,466 patients with medium-high or high bleeding risk were randomised to BioFreedom (polymer-free DES) or BMS with a 1-month DAPT in both cases. The BioFreedom stent arm was better in terms of safety and efficacy. A class IIb recommendation has been made for 1-month DAPT linked to this stent.
Therefore, the ESC guidelines suggest that the withdrawal of DAPT after 3 months in stable patients (class IIa level B) and after 6 months in ACS patients (class IIa level C) in the presence of a high bleeding risk could be considered. The reduction of DAPT to 1 month could be considered in stable cases with increased bleeding risk, but it should be linked to the use of the above-mentioned stent.
A tool that could aid decision making regarding DAPT duration after PCI would be useful and the PRECISE-DAPT score might play a key role. After the input of five simple items, it gives the likelihood of the patient’s out-of-hospital bleeding risk. A cutoff value of >25 could identify those patients at higher risk of bleeding.24
There are several ongoing trials examining the safety of very short-term DAPT in patients with a high-risk of bleeding after new generation DES PCI. These include Bioresorbable Polymer-Coated EES in Patients at High Bleeding Risk Undergoing PCI Followed by 1-Month DAPT (POEM; NCT03112707); Resolute Onyx in One Month Dual Antiplatelet Therapy for High-Bleeding Risk Patients (Onyx-ONE; NCT03344653); XIENCE 28 (NCT03815175 and NCT03355742) and Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated Versus Prolonged DAPT Regimen (MASTER-DAPT; NCT03023020). The results of these trials would enhance the amount of evidence in this setting and would probably change the recommendations in future guidelines.
Role of De-escalation in DAPT
The risk of ischaemic complications is most likely just after PCI and the risk gradually decreases. The same happens after an ACS. Hence, the hypothesis that, during the chronic phase, once the disease stabilises, the level of platelet anti-aggregation required might be lower than in the acute phase. The following trials tested this hypothesis.
The Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment for Acute Coronary Syndromes (TROPICAL-ACS) trial studied patients undergoing PCI after ACS. After 1 week of DAPT with prasugrel and ASA, the participants were randomised to prasugrel or clopidogrel for 12 months. The results of a subsequent platelet aggregation test were used to guide the therapy in the latter group. The results indicate non-inferiority of the de-escalation therapy compared with maintenance therapy.36
The Timing of Platelet Inhibition after Acute Coronary Syndrome (TOPIC) trial involved 645 patients who had a PCI following an ACS, and after an uneventful 1-month period of ASA and a newer P2Y12, were randomised to continue their DAPT regimen or switch to ASA and clopidogrel. After 1 year of follow-up, there were a similar amount of ischaemic events in both groups, with a significant reduction in bleeding complications in the de-escalation group.37
Information on how to de-escalate (switching from prasugrel or ticagrelor to clopidogrel) is found in the 2017 ESC guidelines and in the international expert consensus on switching platelet P2Y12 receptor-inhibiting therapies.12,38
Acetylsalicylic Acid-free Strategy
There is a growing feeling that the era of lifelong ASA treatment might be over, and some studies are trying to assess this topic.
The goal of the Clinical Study Comparing Two Forms of Anti-platelet Therapy After Stent Implantation (GLOBAL-LEADERS) trial was to evaluate 1 month of aspirin plus ticagrelor followed by 23 months of ticagrelor monotherapy compared with 1 year of dual antiplatelet therapy (aspirin plus either clopidogrel or ticagrelor) followed by 1 year of aspirin monotherapy among 15,968 patients undergoing PCI with a BES. The composite outcome, components of the primary outcome and major bleeding were similar between treatment groups. The experimental strategy of a shorter duration of DAPT did not increase ischaemic events.39
The goal of the Short and Optimal Duration of Dual Antiplatelet Therapy After Everolimus-Eluting Cobalt-Chromium Stent-2 (STOPDAPT-2) trial was to evaluate 1 month of DAPT compared with 12 months of DAPT among patients undergoing PCI in stable and unstable settings) with a cobalt chromium everolimus-eluting stent.40 The 3,045 participants were randomised to either 1 month of DAPT followed by clopidogrel monotherapy for 5 years or 12 months of DAPT followed by aspirin monotherapy for 5 years. The authors concluded that 1 month of DAPT followed by clopidogrel monotherapy was superior to 12 months of DAPT followed by aspirin monotherapy at preventing net adverse clinical events (non-inferior at preventing ischaemic events and superior at preventing bleeding).
The SMART-CHOICE trial is also aligned with this ‘off-ASA’ strategy. Other ongoing trials are also assessing ASA-free strategies – Ticagrelor With Aspirin or Alone in High-risk Patients after Coronary Intervention (TWILIGHT; NCT02270242), Ticagrelor Monotherapy After 3 Months in the Patients Treated With New Generation Sirolimus Stent for Acute Coronary Syndrome (TICO; NCT02494895) and Acetyl Salicylic Elimination Trial (ASET; NCT03469856).
DAPT in Patients with Indication for Oral Anticoagulation
It is estimated that about 6–8% of patients who undergo PCI also have an indication to continue chronic therapy with oral anticoagulantion (OAC) due to different pathologies, such as AF, mechanical valvular prosthesis or previous pulmonary embolism. The association of DAPT with OAC increases the risk of haemorrhagic complications up to threefold.
It is especially important for this subgroup of patients to make an adequate stratification of both ischaemic and bleeding risk, as well as the need for chronic anticoagulation. They should follow OAC only when there is a clear indication (CHA2DS2-VASc ≥1 in men and ≥2 in women, mechanical valvular prosthesis, pulmonary embolism or recent or recurrent deep vein thrombosis). The haemorrhagic risk is not static and therefore attention should be paid to the reversible bleeding risk factors present in the different scores or algorithms (HAS-BLED score for major bleeding risk). No predictive risk model developed for patients with OAC has been prospectively validated, so its usefulness in improving the clinical outcomes of patients is questionable.
There are a number of considerations that must be acknowledged to decide the most appropriate approach for these patients.
Type of Antiplatelet Treatment
There are no trials that have assessed the efficacy or safety of triple therapy (DAPT and OAC) with prasugrel or ticagrelor, but there are worrying data showing increased bleeding in various registries. Therefore, it is recommended to avoid ticagrelor and prasugrel in triple therapy.
Duration of Triple Therapy and Triple Versus Dual Therapy
The following trials have evaluated the duration of triple therapy and triple versus dual therapy.
The What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing (WOEST) trial saw 573 patients randomised after PCI to dual therapy with clopidogrel and OAC versus triple therapy with ASA, clopidogrel and OAC for 1 or 12 months (depending on the use of BMS or DES). The dual therapy arm showed a significant reduction in total bleeding and overall mortality without differences in major bleeding or cardiovascular events.41
The Open-Label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention (PIONEER AF-PCI) trial included 2,124 patients with non-valvular AF undergoing PCI. They were divided into three branches: dual therapy with a P2Y12 inhibitor and 15 mg rivaroxaban once daily for 12 months; triple therapy with DAPT plus 2.5 mg rivaroxaban twice daily for 1, 6 or 12 months; and triple therapy with DAPT plus vitamin K antagonist (VKA) for 1, 6 or 12 months. At 12 months, rates of clinically significant bleeding were significantly lower in the two rivaroxaban arms, compared with triple therapy with DAPT and VKA.42
Neither of these studies has sufficient power to assess significant differences in the rate of ischaemic events (stroke or thrombosis).
The Triple Therapy in Patients on Oral Anticoagulation after Drug Eluting Stent Implantation (ISAR-TRIPLE) included 614 patients undergoing PCI receiving OAC.43 They were randomised to receive triple therapy (ASA, clopidogrel and VKA) for 6 weeks or 6 months. There were no significant differences between groups in any aspect.
These three studies consistently show that the rate of bleeding peaks a month after the start of triple therapy and that the rate of haemorrhagic events doubles that of coronary ischaemic events (infarction or thrombosis).
The Evaluation of Dual Therapy with Dabigatran versus Triple Therapy with Warfarin in Patients with AF That Undergo a PCI with Stenting (RE-DUAL PCI) trial included 2,725 patients with AF undergoing PCI. They were assigned to three different arms: at least 6 months of dual therapy with 150 mg of dabigatran twice daily and a P2Y12 inhibitor; at least 6 months of dual therapy with 110 mg of dabigatran twice daily and a P2Y12 inhibitor; and triple therapy with VKA, AAS and a P2Y12 inhibitor for 1 month (BMS) or 3 months (DES), continuing later with dual therapy. The authors found a significant decrease in haemorrhagic events in the two dual therapy arms compared with triple therapy while maintaining safety (not inferiority to VKA).44
A meta-analysis including the four trials mentioned above compared the safety and efficacy of dual versus triple antithrombotic therapy in patients on OAC secondary to AF undergoing PCI. A total of 5,317 patients were included. The study concludes that the dual therapy showed a 47% reduction in TIMI major or minor bleeding with comparable outcomes for MACE.45
The Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation (AUGUSTUS) trial included 4,616 patients with AF and recent ACS or PCI with planned use of P2Y12 inhibitor for at least 6 months. They were randomised in a 1:1 fashion to either apixaban 5 mg twice daily or vitamin K antagonist (VKA) or aspirin 81 mg daily or matching placebo. At 6 months’ follow-up, adding apixaban to a P2Y12 inhibitor resulted in lower rates of bleeding compared with VKA with a lower rate of death or rehospitalisation. The addition of aspirin resulted in greater bleeding without any difference in efficacy in both arms.46
Several other similar trials are currently in the inclusion phase. Of note, the Edoxaban Treatment Versus Vitamin K Antagonist in Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention (ENTRUST-AF-PCI) aimed to assess the role of edoxaban in people with AF following PCI.47 Its results have been recently released. The authors randomised 1,506 patients to either standard triple antithrombotic regimen (VKA-based) or dual antithrombotic therapy with 60 mg edoxaban and a P2Y12 inhibitor. The edoxaban-based dual antithrombotic therapy was non-inferior for bleeding compared with VKA-based triple antithrombotic regimen, without significant differences in ischaemic events.
The ESC guidelines propose two strategies that are selected based on the balance of ischaemic and haemorrhagic risks:
- Triple therapy with ASA, clopidogrel and OAC for 1 month, extendable to 6 months in patients with higher ischaemic risk and lower risk of bleeding.
- Dual therapy with clopidogrel and OAC from the beginning in cases of higher bleeding risk and lower ischaemic risk.
However, the results of REDUAL-PCI and AUGUSTUS trials were known after the publication of the guidelines, and these, along with the other studies mentioned above, seem to suggest the end the use of triple therapy for patients with non-valvular AF undergoing PCI.
The 2018 North American Perspective Update on antithrombotic therapy in patients undergoing PCI recommends a shorter (only periprocedural) period of triple therapy in most settings.48
Definitive Suspension of Antiplatelet Therapy
Data on the appropriate time to discontinue antiplatelet therapy in patients on chronic treatment with OAC are scarce. It is recommended that it is discontinued in patients who have remained stable for 1 year after the PCI, based on studies that show that this strategy is safer.
The Optimizing Antithrombotic Care in Patients With AtriaL fibrillatiON and Coronary stEnt (OAC-ALONE) study compared OAC alone with combined OAC and single antiplatelet treatment among patients with AF beyond 1 year after PCI in a 1:1 randomisation. The primary endpoint was a composite of all-cause death, MI, stroke or systemic embolism. Due to an insufficient sample size (696 patients), the non-inferiority goal was not established. However, the International Society of Thrombosis and Haemostasis stated that major bleeding was lower in the only OAC group, suggesting that OAC alone might be reasonable for patients with AF beyond 1 year after coronary stenting.49
Dual therapy with an antiplatelet drug and an OAC is limited for patients who meet some criterion which indicate they have a high risk of ischaemia, such as previous stent thrombosis, PCI of the only permeable coronary artery, diabetes with diffuse coronary disease, renal insufficiency, more than three stents implanted, more than three lesions treated, bifurcation treated with two stents technique, total length of stent >60 mm or treatment of a chronic occlusion.
Type of Stent
The choice of a new generation DES over a BMS in patients with OAC indication is well established.
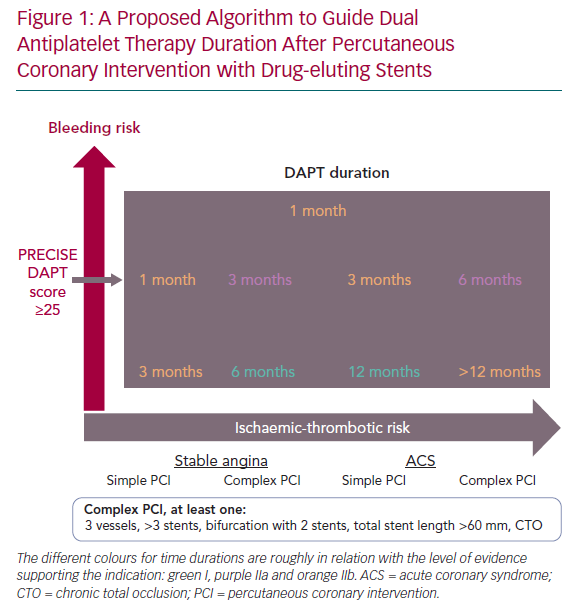
We have developed an algorithm at the Hospital Universitario Marqués de Valdecilla that we propose as a tool to help decide the duration of DAPT after PCI (Figures 1 and 2).
There are two different algorithms depending on the need of chronic anticoagulation. The protocols consider the bleeding risk assessed with the PRECISE-DAPT score and the presence of certain comorbidities as well as the ischaemic risk assessed with the clinical presentation and the PCI complexity. We consider a PCI to be complex when at least one of the following is present:
- PCI over three vessels.
- The use of more than three stents.
- A two-stent strategy in a bifurcation
- Total length of stent >60 mm.
- The treatment of a chronic total occlusion.
The different colours for time durations are roughly related to the level of evidence supporting the indication. It is designed to guide the clinician in the final decision, and it is meant to be thought of as a continuum.
Conclusion
PCI has evolved dramatically in recent years and it has changed the approach in the management of ischaemic heart disease. This has been due to the development of new devices and antithrombotic therapies. We have reviewed the indications and modalities of antiaggregant-anticoagulant therapy after PCI in different scenarios. The multiple trials carried out have modified the approach of this therapy from a routine general approach that did not consider individual variables of the patients to a more comprehensive approach that takes into account the balance of ischaemic-thrombotic and haemorrhagic risks, informed from patient variables, coronary heart disease symptoms and features of the PCI procedure.