Atrial fibrillation (AF) is the most common clinically-significant arrhythmia in the world.1 It is estimated that, in the US alone, approximately 2.5 million people have AF, with the condition being 1.5 times more common in men than in women.2 Despite the decline in morbidity and mortality from cardiovascular diseases in general due to advances in prevention and treatment, AF has not followed a similar trend. Over the coming years, the incidence of AF is expected to increase.3
AF is a well-established risk factor for other cardiovascular diseases, including ischaemic stroke and heart failure.4 Growing evidence, however, indicates that AF can have deleterious effects beyond an increased risk of cardiovascular diseases. Specifically, many recent studies have explored the impact of AF on cognition and dementia risk. With the ageing population, the burden of dementia is expected to increase globally. More than 20 % of people aged >70 years have mild cognitive impairment (MCI).5 Approximately 800,000 individuals develop MCI and >500,000 develop dementia annually in the US.6 The total number of new cases of dementia each year worldwide is about 7.7 million, which means a new case is diagnosed every 4 seconds. It is estimated that 35.6 million people worldwide were living with dementia in 2010, and this figure is expected to double every 20 years, reaching 65.7 million in 2030 and 115.4 million in 2050.7 A better understanding of the association of AF with dementia and cognitive impairment, the predictors of cognitive impairment among those with AF, and the potential mechanisms explaining such associations would inform strategies for the management of AF and the prevention of adverse cognitive outcomes among these patients. We conducted a review of the literature to examine the current evidence supporting an association of AF with cognitive function, dementia and MCI; describe the predictors of cognitive outcomes in people with AF; summarise the potential pathophysiological mechanisms; discuss the preventive interventions specific for AF; and explore the potential impact of current AF treatments on cognitive decline.
To inform this review, we searched PubMed for publications available as of 1 June 2016, using the search query [atrial fibrillation AND (dementia OR cognitive impairment OR cognitive decline)], which considered the previous terms in any field as well as occurring as MeSH terms. We considered publications mostly from the past 5 years (since 1 November 2010), although we did not exclude frequentlyreferenced older publications and selected those considered relevant.
AF and Cognitive Function
The most basic evidence supporting an association of AF with worse cognitive function comes from cross-sectional studies comparing cognition in individuals with and without AF. A major limitation of these studies, however, is the difficulty in discerning the temporality of the association. In a study in Germany including 122 stroke-free individuals with AF and 564 individuals without AF undergoing a detailed cognitive assessment, those with AF performed significantly worse in learning, memory and executive function tasks.8 Similarly, the prevalence of AF has been associated with amnestic MCI and impaired global cognitive function in cross-sectional studies in Europe and the US.9,10 Interestingly, a recent analysis of the US-based Atherosclerosis Risk in Communities (ARIC) study, which included 325 individuals who underwent detailed cognitive assessment and heart rhythm monitoring during a maximum of 14 days, found that persistent but not paroxysmal AF was associated with lower cognitive function.11 These findings suggest that AF burden, in addition to its presence, may influence cognitive function.
AF and Cognitive Decline
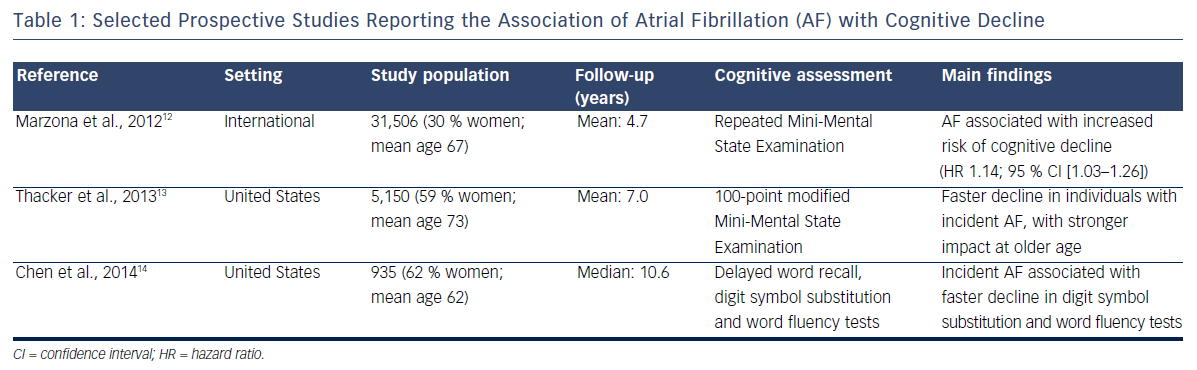
Cross-sectional studies have methodological problems that limit the interpretation of their results. Longitudinal studies with repeated assessment of cognitive function provide a more rigorous appraisal of the association of AF with cognition. Table 1 presents the summary characteristics of selected prospective studies. An analysis of 31,506 participants in the international Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) and Telmisartan Randomised Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (TRASCEND) trial followed up for a median of 5 years found that those who had AF at baseline or developed it during the study had a 13 % increased risk of cognitive decline, defined as a decrease of three or more points in the Mini- Mental State Examination.12 Similar findings were reported in the Cardiovascular Health Study, a community-based study in the US in which the development of AF was associated with faster decline measured by modified Mini-Mental State Examination score during a mean follow-up of 7 years.13 More recently we found that, among 935 stroke-free participants in the ARIC Study, incident AF was associated with a faster decline in measures of executive function and verbal fluency. This association was only present among individuals with subclinical cerebral infarcts, suggesting that vascular disease may mediate the link between AF and cognitive decline.14
AF and Prevalence of Dementia and Mild Cognitive Impairment
In addition to exploring how AF affects cognitive trajectories over time, it would be useful to determine how incident AF is associated with the onset of dementia and MCI. It would be particularly useful to assess whether such association is independent of clinical stroke, since this would point to additional pathways linking AF and neurodegeneration.
A few cross-sectional studies have explored differences in the prevalence of dementia/MCI by AF status. Investigators from the Rotterdam study, which included 6,584 participants aged 55 and older, were among the first to report an association between AF and dementia/cognitive impairment. In this community-based prospective cohort in Ommord, a suburb of Rotterdam in the Netherlands, the prevalence of dementia was more than double in those with compared to those without AF. The association was stronger in women and younger (<65 years of age) participants. A history of stroke among those with AF was not enough to account for the association, supporting the presence of alternative mechanisms.15 More recent studies conducted in smaller cohorts have replicated these associations, demonstrating a higher prevalence of both Alzheimer’s disease-type and vascular dementia among individuals with AF compared to those without AF, independent of potential confounders.16,17
AF and Incidence of Dementia
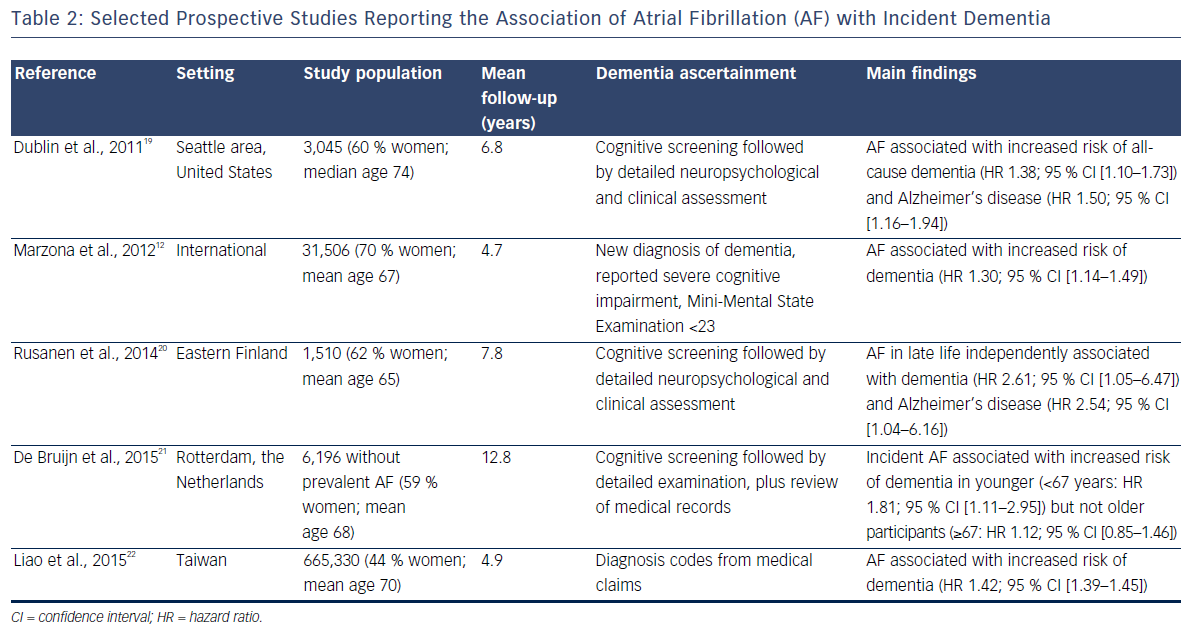
The elevated incidence of stroke in AF patients explains the relationship between AF and the development of vascular dementia.18 More recently, several prospective studies have shown that AF is also linked with an elevated risk of other dementias, including Alzheimer’s disease, independent of clinical vascular events. Table 2 provides the summary characteristics of relevant studies.
An analysis of 3,045 community-dwelling individuals in the Seattle area of the US found that the development of AF was associated with a 50 % increase in the risk of receiving a diagnosis of Alzheimer’s disease. This association was present in individuals with and without clinically-recognised stroke.19
Increased risk of dementia associated with AF has been reported in a secondary analysis of the ONTARGET and TRASCEND trials,12 the Finnish Cardiovascular Risk Factors, Aging and Dementia (CAIDE) study20 and the Rotterdam cohort.21 Further confirmation of this association has come from an analysis of large administrative databases in Taiwan, including >600,000 individuals. This analysis showed a 42 % increased risk of dementia in those with AF versus those without AF.22 A recent systematic review and meta-analysis of previously published studies on the topic reported a hazard ratio (95 % confidence interval) for dementia of 1.42 (1.17–1.72) when comparing individuals with AF to those without AF.23
AF and Brain Abnormalities
Evaluating the impact of AF on the prevalence and development of brain abnormalities can help advance our understanding of the mechanisms linking AF with cognitive decline and dementia. Several reports emphasise the presence of signs of cerebrovascular disease in the brains of patients with AF. Overall, AF patients have a higher burden of silent cerebral infarcts and white matter disease, and may have an increased prevalence of cerebral microbleeds.24 These lesions may explain the fast cognitive decline and elevated dementia risk among AF patients.14 The presence of these abnormalities may also have an impact on the management of AF patients, for example influencing decisions about whether or not to prescribe oral anticoagulation.25
Several recent studies have explored the impact of AF on other brain-related phenotypes. In the Icelandic population-based Age, Gene/Environment Susceptibility–Reykjavik Study, which included 4,251 participants, AF was associated with lower brain volume and grey matter.26 Similar findings have recently been reported in a cross-sectional study in individuals from a community-based study in Minnesota, USA.27 An autopsy study including 134 individuals with and 194 without AF found that the prevalence of neuropathological changes (neuritic plaques, neurofibrillary tangles) associated with Alzheimer’s disease was higher in individuals with permanent AF than in those without AF.28
Predictors of Cognitive Impairment and Dementia in People with AF
Understanding the determinants of dementia among AF patients may inform interventions that can prevent the cognitive complications of the arrhythmia. Some studies have found that higher CHADS2 and CHA2DS2-VASc scores, which are stratification schemes commonly used to inform anticoagulant treatment in individuals with AF, predict dementia in these patients.22,29 This association is not surprising given that age, possibly the strongest predictor of dementia, is part of the scores. Dementia-specific risk models are likely to provide more accurate predictions.
Oral anticoagulation is a mainstay of the treatment of patients with AF. Determination of the influence of anticoagulation control in AF patients on the risk of dementia has been the objective of at least two separate publications. Both studies used data from the Intermountain Healthcare Clinical Pharmacist Anticoagulation Service in Utah, USA. They reported that vitamin K antagonist users whose levels were only within the therapeutic range for a low proportion of time were at a higher risk of dementia due to under- or overcoagulation.30,31 Though informative, these studies are limited in that they cannot determine whether baseline cognitive function confounds the association between suboptimal oral anticoagulation and the future risk of dementia. For example, individuals with worse cognitive function at the time of oral anticoagulation initiation may have more problems following an adequate therapeutic regimen and would be at a higher risk of being diagnosed with dementia later on. Additional observational studies with adequate characterisation of baseline cognition or, even better, randomised trials aimed at improving anticoagulation quality are needed to answer this question.
Mechanisms Linking AF, Cognitive Decline and Dementia Risk
The published evidence is consistent in supporting an association between AF and cognitive outcomes. The mechanisms underlying this association, however, still need clarification. An obvious pathway linking AF with cognitive decline or dementia is the elevated risk of stroke. AF is associated with at least a doubling of stroke risk32 and the effects of stroke on cognitive function are well established.18 Despite this, elevated stroke risk does not completely mediate the increased risk of dementia and cognitive decline associated with AF.33 Other mechanisms such as silent cerebral infarcts, microbleeds associated with oral anticoagulation and cerebral hypoperfusion are likely to play a role (Figure 1).
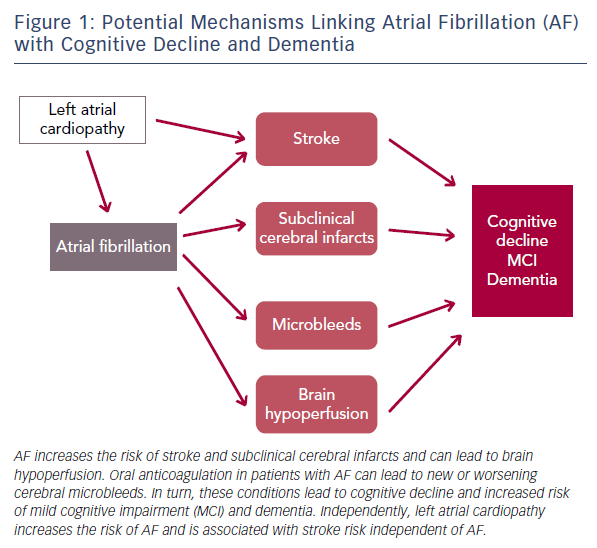
AF more than doubles the risk of silent cerebral infarcts independent of stroke34 and the presence of silent cerebral infarcts is a risk factor for dementia.35 At least one study has specifically addressed the role of subclinical cerebrovascular disease as a mediator of the association between AF and cognitive impairment. In a subset of stroke-free participants in the ARIC study who underwent repeated brain magnetic resonance imaging after approximately 12 years, we showed that AF was only associated with cognitive decline in those who had developed incident silent cerebral infarcts.14 The hypercoagulable state resulting from AF is certain to play a role in this mechanism and, consequently, anticoagulation may be effective in preventing adverse cognitive outcomes in patients with AF.36
Similarly, AF could increase dementia risk through its impact on cardiac function. Patients with AF have been found to have reduced cerebral perfusion;37 and restoration of sinus rhythm in AF patients through cardioversion or ablation leads to improvements in cerebral blood flow.38,39 Reduced diastolic function and low cardiac index, both potential consequences of AF, have been associated with incident dementia in prospective studies.40,41 Moreover, AF is an established risk factor for heart failure,42 which in turn can worsen cerebral hypoperfusion.43
Microbleeds may also explain part of the association of AF with cognitive impairment. These brain lesions are relatively frequent and have been linked with an increased risk of cerebral haemorrhage, lacunar infarcts and degenerative changes in brain matter.44 Oral anticoagulation in persons with AF can increase the risk of developing microbleeds or worsen the impact of existing microbleeds on cognitive function. In the community-based Rotterdam study, individuals using coumarin anticoagulants had a higher prevalence and incidence of microbleeds. This risk was notably higher among individuals with a greater variability in anticoagulation control.45
Of recent interest is the potential role that left atrial cardiopathy, as a precursor of AF, can play in cerebrovascular disease and, consequently, the development of cognitive decline and dementia. Analysis of several community-based studies has demonstrated that the presence of electrocardiographic left atrial abnormality, a marker of atrial cardiopathy, is associated with an increased risk of ischaemic stroke, mostly non-lacunar, and vascular brain injury even in the absence of AF.46,47 The impact of left atrial cardiopathy on dementia risk independent of AF needs to be explored.
Prevention of Cognitive Impairment in People with AF
Understanding the mechanisms responsible for the increased rates of cognitive decline and dementia in people with AF can inform preventive strategies. Current guidelines recommend oral anticoagulation for stroke prevention in most individuals with AF.48,49 The decreased stroke risk in patients receiving adequate anticoagulation should consequently lead to a reduced risk of adverse cognitive outcomes. Improved anticoagulation control could be particularly effective in high-risk individuals, for example those who already have some cognitive impairment. A recent clinical trial including 973 elderly patients with AF found that patients randomised to warfarin had less cognitive decline than those randomised to aspirin after 33 months of follow-up, although the differences were not statistically significant.50 A recent analysis comparing the risk of dementia in patients with AF using warfarin versus non-vitamin K oral anticoagulants reported a lower risk of dementia among non-vitamin K oral anticoagulant users.51 Unfortunately there is currently no additional evidence on the effect of oral anticoagulation on cognitive function as larger randomised trials of oral anticoagulation, whether traditional vitamin K antagonists or the more recent direct oral anticoagulants, have not considered cognitive endpoints. The long-term cognitive effects of other treatments for AF, such as catheter ablation, are unknown. Future studies that collect prospective information on cognitive outcomes should address this gap.
A promising novel area in the management of AF, which could eventually translate into the prevention of cognitive complications, is the role of lifestyle changes leading to weight loss and improvement in overall cardiometabolic risk profile. A randomised trial of weight loss and risk factor management in 150 AF patients led to clinicallysignificant reductions in AF burden and symptoms as well as improving cardiac function.52 Similar findings were obtained in 355 AF patients participating in a weight loss intervention; those who sustained weight loss had reductions in AF burden and were more likely to remain in sinus rhythm.53 The impact of these lifestyle interventions on cognitive outcomes in persons with AF has not been assessed to date. Given the role of cardiovascular risk factors in cognition and dementia risk,54 this area would be a fruitful avenue for future research.
Finally, primary prevention of AF should be the ultimate goal in reducing the burden of AF-related complications. Unfortunately, we currently lack effective interventions that have consistently demonstrated effectiveness in reducing AF risk in the general population. Although promising, some preventive interventions such as omega-3 fatty acid supplementation, statins or inhibition of the renin–angiotensin–aldosterone system have failed to reduce AF risk.55,56 Recent studies indicate that dietary intervention and improved blood pressure control can prevent AF.57,58 Whether these interventions can in turn lead to a reduced risk of dementia and cognitive decline remains undetermined.
Conclusion
A growing and consistent body of literature supports AF as a risk factor for cognitive decline and dementia. The mechanisms responsible for this association are diverse and go beyond the wellestablished increase in stroke risk in individuals with AF. Future research needs to deepen the understanding of these mechanisms and, more importantly, develop interventions that reduce the burden of adverse cognitive outcomes associated with AF.







