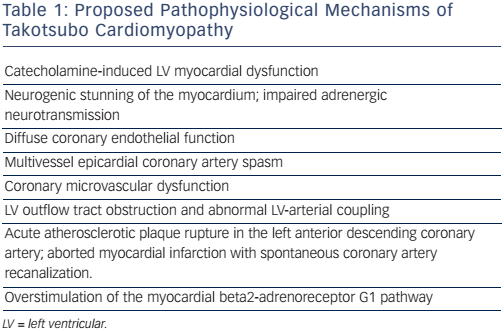
Takotsubo syndrome (TTS) is an acute, profound, but reversible heart failure syndrome, usually but not always triggered by physical or emotional stress. To date, the exact pathogenic mechanism of this syndrome remains unclear; however, several hypotheses involving vascular mechanisms (i.e. abnormal coronary epicardial or microvascular vasoreactivity),1-3 endocrine and gender-related mechanisms (i.e. oestrogen deficiency),4 and both autonomic and central nervous system abnormalities involving catecholamine surges,5,6 have been proposed, suggesting a multifactorial pathogenic background (see Table 1).
Although practically indistinguishable from an acute coronary syndrome at clinical presentation, some characteristics of TTS, such as the involvement of a myocardial area that extends beyond a single coronary vessel territory, the complete recovery of ventricular function in days or weeks, and the absence of significant epicardial coronary artery disease, make TTS a unique form of acute heart failure syndrome. The diagnosis of TTS has been traditionally based on the proposed Mayo Clinic criteria.7 A more recent report by the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology has suggested newer diagnostic criteria that include: (a) transient regional wall motion abnormalities of LV or RV myocardium, which are frequently, but not always, preceded by a stressful trigger (emotional or physical); (b) regional wall motion abnormalities usually extend beyond a single epicardial vascular distribution, and often result in circumferential dysfunction of the ventricular segments involved; (c) absence of culprit atherosclerotic coronary artery disease including acute plaque rupture, thrombus formation, and coronary dissection or other pathological conditions to explain the pattern of temporary LV dysfunction observed (e.g. hypertrophic cardiomyopathy, viral myocarditis); (d) new and reversible electrocardiography (ECG) abnormalities (ST-segment elevation, ST depression, left bundle branch block (LBBB), T-wave inversion, and/or QTc prolongation) during the acute phase (3 months); (e) significantly elevated serum natriuretic peptide (BNP or NT-proBNP) during the acute phase; (f) positive but relatively small elevation in cardiac troponin measured with a conventional assay (i.e. disparity between troponin level and amount of dysfunctional myocardium present); and (g) recovery of ventricular systolic function on cardiac imaging at follow-up (3–6 months).8
Advanced imaging modalities are becoming increasingly important in the management of TTS, providing essential information for the diagnosis and prognosis of these patients. Cardiac magnetic resonance, in particular, has become a first-line modality for non-invasive assessment of patients with TTS. In addition to a comprehensive assessment of the myocardial functional and anatomical properties, cardiac magnetic resonance (CMR) is uniquely suited to differentiating TTS from other forms of acute ventricular dysfunction associated with patent coronary arteries. In this setting CMR may have a major impact on both clinical and management and risk stratification. This review focuses on the emerging role of CMR in the diagnosis, clinical evaluation and risk stratification of TTS patients.
Cardiac Magnetic Resonance in Takotsubo Syndrome
Cardiac magnetic resonance imaging has emerged as a first-line diagnostic tool for the assessment of TTS. In addition to visualizing the hallmark regional wall motion abnormalities that characterize the syndrome, CMR can identify the presence of reversible and irreversible myocardial damage (i.e. myocardial oedema and scarring, respectively). CMR can also identify potential complications of TTS, such as LV outflow tract (LVOT) obstruction, valve disease, pericardial effusion and LV thrombus.
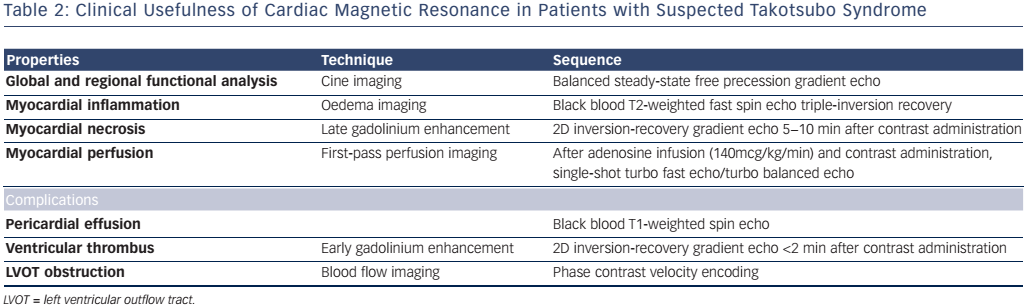
Steady state-free precession cine, phase contrast sequences, black-blood T2-weighted triple inversion recovery, first-pass perfusion, early gadolinium enhancement (EGE) and late gadolinium enhancement (LGE) sequences are the most widely used sequences in TTS. Other CMR techniques, such as T1 and T2 mapping techniques and feature/ tissue tracking, could be potentially contributive, though at present are used only for research purposes. Table 2 summarizes the CMR tools and information acquired in the setting of suspected TTS.
Left Ventricular Function and Wall Motion Abnormalities
One of the hallmarks of TTS is the presence of a reversible systolic impairment of LV function affecting mainly the LV apex. This regional wall motion abnormality extends beyond a single epicardial vascular distribution. Typically, a mid-cavity to apical ballooning with sparing of the LV basal segments occurs. However, other contraction patterns during the acute phase of TTS including mid-ventricular, basal and focal forms may be identified in up to 18 % of the cases.9 Complete recovery in 96 % of patients occurs within 7−37 days. It has been observed that typical and mid-ventricular TTS forms have more severe heart failure symptoms.10
With its high spatial resolution, CMR is the modality of choice for the assessment of ventricular function as well as regional wall motion.11 For structural abnormalities, free precession breath-hold cines in short and long axis and outflow tract are useful. The typical form presents with contraction abnormalities in the ventricular wall, equally affecting the anterior, inferior, and lateral walls, that extend beyond a single epicardial vascular territory. Another finding suggestive of TTS is the presence of basal segments hyperkinesia, contributing to the characteristic morphology.
Cardiac magnetic resonance may efficiently demonstrate biventricular involvement and correlate the functional pattern to the tissue properties of the myocardium. In a population of 31 TTS patients presenting predominantly with ST-elevation electrocardiogram, RV functional involvement was identified in half, with associated myocardial oedema in both functioning and malfunctioning segments, persisting for up to 4 months following the acute presentation, as measured by repeatedly abnormal native T1 mapping and increased extracellular volume measurements.12
With the use of left atrial (LA) functional indexes, CMR allows additionally for the assessment of the atrial function. Compared to anterior STEMI patients, TTC patients demonstrated a significantly decreased LA function as assessed by CMR volumetric indexes derived from fractional volume changes during the acute phase of the disease. However, impairment of LA performance seems to be transient in TTS with full recovery during follow-up.13
Myocardial Inflammation
Myocardial inflammation is a key tissue property in the acute phase of TTS, which was been shown to be reversible at follow-up.14 Hallmark changes in inflammation include myocardial hyperaemia, oedema and fibrosis, all of which can be non-invasively visualized using CMR imaging.15 The area of oedema corresponds to the area of wall motion abnormalities.16 The pathophysiological substrate explaining myocardial oedema in these patients has not been elucidated, even though the presence of inflammatory phenomena and transient ischaemia appear to play a pivotal role.
T2-weighted CMR is the standard imaging technique for detecting acute myocardial oedema in vivo.17,18 It is used extensively in the study of myocardial inflammation in acute and chronic myocarditis,15 as well as in the setting of ischaemic heart disease, for the differentiation of acute from chronic MI19 and for assessment of the area at risk.20 When global myocardial involvement is suspected, a quantitative approach is preferred, classically by calculating the SI ratio between myocardium and skeletal muscle. Parametric imaging (T1- and T2-mapping) is an emerging and more robust technique that provides a quantitative means to detect myocardial edema.21 Each tissue type exhibits a characteristic range of normal T1 and T2 relaxation times at a particular magnetic field strength.
Typically, T2-weighted sequences in TTS patients identify the presence of circumferential, transmural oedema of the apical to mid-cavity myocardium matching with the regional wall motion abnormalities.16 Myocardial oedema is a transient dynamic phenomenon, normally progressively resolving between 3 and 6 months. CMR has an excellent diagnostic accuracy in detecting myocardial inflammation in TTS. In a multicentre study by Eitel et al.,22 myocardial oedema was seen in 81 % of patients, whereas patchy delayed gadolinium enhancement was seen in 9 % of TC patients. Neil et al. found increased T2-weighted signal intensity, particularly at the apical cavity in all TTS patients, which persisted after 3 months and was inversely related to myocardial strain.23 Nakamori et al. monitored 23 TTS patients.24 Upon presentation, myocardial oedema on T2-weighted MRI was observed in 96 %, and LGE was observed in 22 %. All instances of LGE were observed in an area that exhibited both wall motion abnormalities and oedema in a transmural distribution.
In accordance with the above-mentioned data, myocardial oedema on CMR is a constant feature in TTS, reflecting acute inflammation.
Myocardial Necrosis
In principle, myocardial oedema resolves without any myocardial scarring and with complete functional recovery (see Figure 1). The importance of detecting LGE, which reflects myocardial damage, in TTS patients relies on the observed association between the presence of LGE and poorer prognosis in both ischaemic and non-ischaemic cardiomyopathies.25 Early CMR suggested that lack of LGE was a necessary condition to diagnose TTS,26 and would thus help differentiate it from MI, where LGE is always present to some degree, and myocarditis, where 88 % of patients show a patchy type of LGE.27
To assess for the presence/absence of scarring tissue, 10 min after contrast injection, short and long axis acquisitions identical to the ones done in cine mode, are performed using an inversion-recovery gradient echo sequence.28 In general, LGE indicates the relative changes of the extracellular and intracellular volumes. In contrast, T1 mapping sequences provide a different approach to tissue characterization, allowing for the measurement of intrinsic relaxation times of myocardium pre- and post-gadolinium infusion, not dependent on relative signal intensities.29
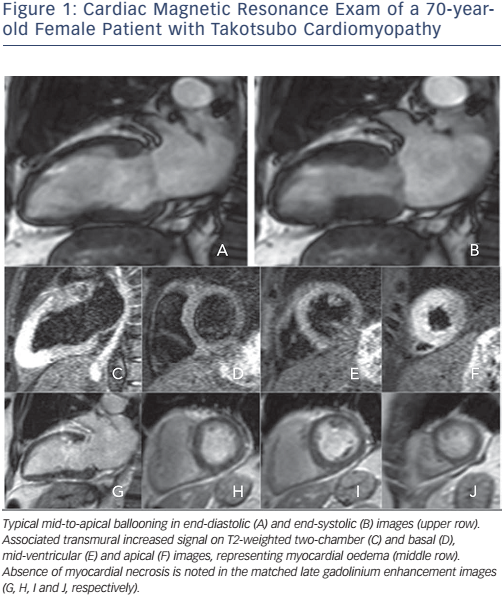
Some isolated reports30,31 and, more recently, more significant studies,22,32,33 showed that LGE can happen in the context of TTS. However, experimental data show that delayed washout of gadolinium may be caused by increased interstitial water content associated with transient myocardial oedema, rather than irreversible myocardial necrosis/fibrosis.24
Rolf et al. performed multiple endomyocardial biopsies in sites with and without LGE in the acute phase and after recovery.34 All patients with TTS showed a significant increase of collagen-1 compared with control tissue. Moreover, the amount of collagen-1 was significantly higher in LGE-positive patients. There were no differences in the extent of oedema on T2-weighted measurements between groups. Sacha et al.35 and Maréchaux et al.36 have previously reported cases of contraction-band necrosis detected at autopsy, a possible explanation for LGE in patients with TTS. Broad evidence on the long-term (reversible or irreversible) presence of LGE and its prognostic value is lacking.
Myocardial Perfusion
There is a growing body of evidence strongly implying a major role of microvascular dysfunction (MVD) in the pathogenesis of TTS. Invasive studies (using thrombolysis in myocardial infarction frame counts, myocardial perfusion grading, intracoronary Doppler evaluation of vasomotor function, coronary flow reserve and thermodilution) and non-invasive studies (using Doppler echocardiography, myocardial contrast echocardiography and SPECT/PET metabolism/perfusion) have all demonstrated coronary microvascular dysfunction.3,37-40 The above data suggest abnormal perfusion properties within dysfunctional myocardial area in the acute phase, recovered at follow-up. According to the most accepted mechanism of TTS, which proposes a vigorous neurohumoral discharge precipitated by emotional stress, severe microvascular dysfunction could be expected, leading to secondary myocardial stunning. However, whether MVD is the causative factor or a consequence of this disorder remains elusive.
Although perfusion MRI is a well-established exam for the assessment of epicardial41 and microvascular42 ischaemia, there is practically no evidence of the use of perfusion CMR in TTS. Perfusion CMR, in particular with the use of quantitative techniques, may offer significant knowledge on the pattern of MVD in the acute and chronic phase of the disease.
Complications
Possible complications in TC include left heart failure with or without pulmonary oedema, acute pericarditis, mitral valve regurgitation, ventricular arrhythmias, intramural thrombus, stroke, LV free wall rupture35 and death.9 In addition to the accurate assessment of the cardiac function with cine imaging as well as the anatomical study of the myocardium, pericardium and valves, CMR offers accurate assessment of any intracavitary thrombus.43 With the use of EGE sequence, performed within 2 minutes after contrast agent infusion, a thrombus appears as a low signal intensity (no gadolinium uptake), nearly black, which makes contrast with the intermediate signal of myocardium and blood pool.
Risk Stratification
Although TTS is widely considered as a benign reversible condition, its associated risk for major cardiac events in the acute and chronic phase is increasingly recognized.44 In fact, TTS represents an acute heart failure syndrome with substantial morbidity and mortality, comparable to patients with acute coronary syndromes. Physical triggers, acute neurologic or psychiatric diseases, high troponin levels and a low ejection fraction on admission are recognized as independent predictors for in-hospital and long-term adverse major cardiac events.
The clinical spectrum of TTS is considerably broad and a prospective study evaluating a precise CMR pattern related to outcome is lacking.
In the largest multicentre study in this field enrolling 239 consecutive patients with CMR at initial presentation, 1- and 6-month follow-up, a moderate-to-severe LV function reduction in all patients in the acute phase with associated myocardial oedema in 81 % and focal or patchy LGE in 9 % was identified. During follow-up, four patients died. In the remaining patients, there was full resolution of the above abnormalities. There was no relation between the occurrence of LGE and clinical presentation, mortality, age, sex, ECG pattern, or type of stress trigger.22 Naruse et al. reported, in a cohort of 20 patients with TTS, no significant differences in age, sex, congestive heart failure, peak creatine kinase, noradrenaline elevation, ECG abnormalities, ejection fraction, type of TTS and time from onset of symptoms to CMR in the LGE positive group.32 However, there was a greater prevalence of cardiogenic shock and longer time to normalize ECG and echocardiographic changes.
The incremental prognostic value of LGE in TTC is yet to be elucidated. Although LGE is widely considered to be a marker associated with an increased frequency of cardiogenic shock, longer duration to resolution of wall motion abnormalities there are no large-scale studies so far to address this hypothesis. In a small CMR study including twenty TTS patients with a mean 11±9 months follow-up, the presence of LGE, reflecting irreversible myocardial damage, was not associated with adverse long-term outcomes as compared to patients with intact myocardium.45
Differential Diagnosis
As patients with TTS commonly present with symptoms similar to those among patients with an acute coronary syndrome, initial diagnosis and treatment of patients in the emergency setting remains challenging.
In one study, troponin levels and electrocardiographic changes on admission were not sufficient to differentiate between these two disorders, as more than 80 % of patients with TTS had elevated troponin levels and nearly 80 % had signs of myocardial ischemia on initial electrocardiography.46 Notably, in another study examining the angiographic results in TTS patients, 15.3 % of patients had evidence of coexisting coronary artery disease on angiography, thus demonstrating that the presence of coronary artery disease does not preclude the diagnosis of the syndrome.47
The combination of typical regional wall motion abnormalities with matching reversible myocardial oedema and no or subtle delayed enhancement may serve as a very useful set of diagnostic criteria in cases with myocardial infarction with non-obstructive coronary artery disease (MINOCA),48 helping in particular to distinguish TTS from embolism and myocarditis. Preliminary data demonstrate that in 125 patients with a presumed diagnosis of TTS examined with CMR cine and LGE imaging, 29 % were ultimately diagnosed with apical infarction.49
Conclusion
CMR is a first-line modality for the assessment of TTS, offering a comprehensive, safe and reproducible assessment of the myocardial functional and anatomical properties, as well as of the potential complications of the syndrome. This information allows determination of diagnosis and differentiation from other acute entities with similar clinical presentation. Further large-scale studies are required to elucidate the exact clinical and prognostic value of CMR parameters in the management of the disease.