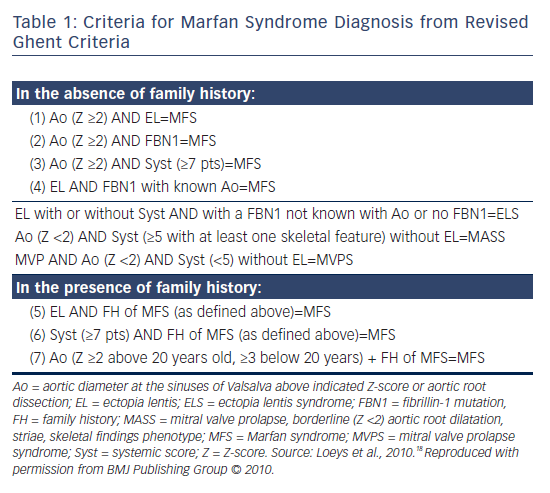
Marfan syndrome (MFS) is a disease in which connective tissue becomes weak secondary to fibrillin-1 mutations, resulting in aortic dilatation, aneurysm formation, aortic dissection, aortic regurgitation and mitral valve prolapse (MVP; see Table 1).
Epidemiology
MFS is an autosomal dominant condition: 75 % of all patients inherit the condition from one affected parent and 25 % are affected as the result of a new mutation. The population incidence is 2–3 per 10,000.1 The autosomal dominant inheritance of this disorder was described in 1934,2 and has been ascribed to abnormalities in fibrillin-1 protein (discovered in 1990),3 which is encoded by the FBN1 gene (reported in 1991).4 The most common causes of death in MFS are cardiovascular, especially aortic dissection and rupture. According to a Taiwanese study, aortic dissection is the most serious complication, occurring in 9.7 % of individuals with MFS (nearly 61 % of these patients are male) and carrying an average mortality of approximately 10.6 %.5
Cardiovascular Manifestations
In MFS, the main cardiovascular manifestations are aortic dilatation and MVP. Tricuspid regurgitation (TR), pulmonary artery (PA) dilatation, ventricular arrhythmia and dilated cardiomyopathy also occur.
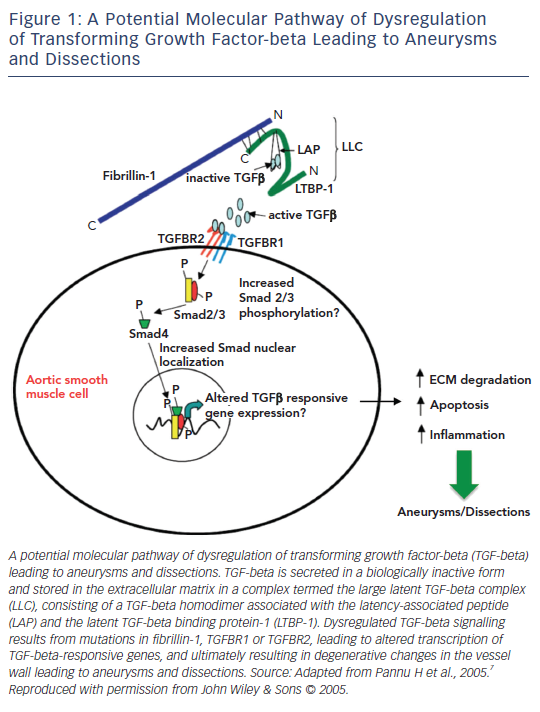
Pro-transforming growth factor-beta (TGF-beta) binds to the latent TGF-beta binding protein-1 (LTBP-1) and forms the latency-associated peptide (LAP), followed by a complex termed the large latent TGF-beta complex (LLC). This is secreted and sequestered in the extracellular matrix (ECM). FBN1 is a matrix glycoprotein and the major constituent of ECM microfibrils comprising elastic fibres. In MFS, the ECM is not normal and when the ECM is damaged due to the force of the blood flow ejected from the heart, the mesenchymal cells promote active TGF-beta to restore the ECM. This results in excessive TGF-beta signalling, causing ECM degradation, apoptosis and an inflammatory state, leading to aneurysm formation or dissection (see Figure 1).6,7
At present, more than 3,000 FBN1 mutations have been discovered. Almost all of them develop similar manifestations such as heart, eye and skeletal problems, and are related to excessive TGF-beta signalling via integrin, which provides a common mechanism by promoting latent TGF-beta and expressing TGF-beta.6
Endothelial cells, smooth muscle cells and fibroblasts sense and respond to blood flow and blood pressure. Increasing or decreasing blood pressure increases or decreases wall stress. Cells sense and regulate the ECM through integrins and cytoskeletal components. Sensing high versus low stress causes different cell signalling. Misperception of high stress as low stress can cause maladaptive remodelling by activating the pathways observed in thoracic aortic aneurysms and aortic dissections (TAAD).8
The Aorta
In MFS, aortic dilatation and aneurysm formation are caused by cystic medial necrosis, in which the medial layer of the aorta demonstrates fewer cells and a lacunar appearance. Most aortic dilatation starts in the sinuses of Valsalva. A reduced content of elastic fibres (including fibrillin-1) associated with continual force from left ventricular (LV) cyclic torsion applied to the aortic root are thought to be the main reasons why dilatation starts at the aortic sinus.9 Aortic root dilatation is the most common cardiovascular manifestation occurring in 60–80 % of MFS patients,9 and aortic sinus enlargement causing aortic aneurysm occurs in 50–60 % of adult patients and 50 % of paediatric patients.10 Syndromic thoracic aortic aneurysm (TAA) growth rate is variable in each TAA subtype. The average rate of TAA growth in MFS patients is 0.5–1.0 mm per year.11 Ascending aortic dilatation increases with age and 96 % of patients have ascending aortic dilatation by 60 years.12
In comparison, the average rate of TAA growth in patients with Loeys–Dietz syndrome, a similar but more serious cardiovascular disorder, is more than 0.5–1.0 mm per year.13,14
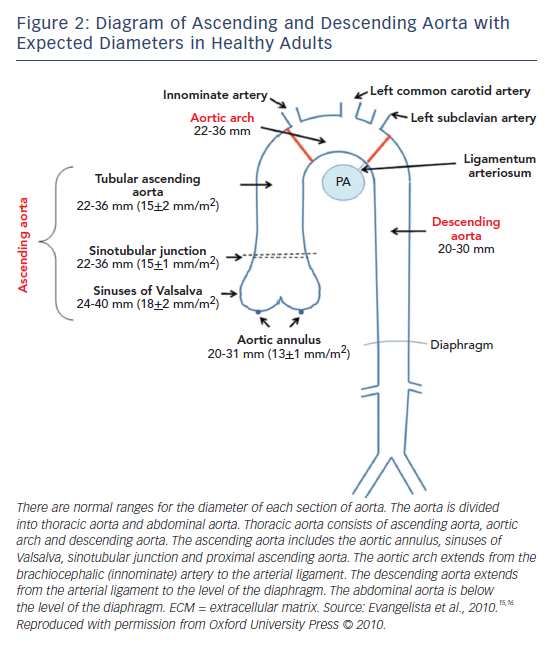
Figure 2 depicts the normal dimensions of the ascending and descending aorta in healthy individuals.15,16 Aortic size is strongly influenced by body surface area (BSA), weight, age and sex.17 When the aorta dilates, the risk of aortic dissection/rupture becomes higher. In MFS, aortic diameter is used for monitoring, but its significance is influenced by BSA, so the Z-score, which is adjusted to BSA and age, is used.18,19 In MFS, the average speed of aneurysm growth in the ascending aorta is 0.5–1.0 mm per year and after aortic root replacement for aortic dissection, is 0.58 ± 0.5 mm per year in the distal descending aorta.20 The distal aorta can be the first site of dissection or prophylactic surgery in up to 18 % of patients with MFS.21
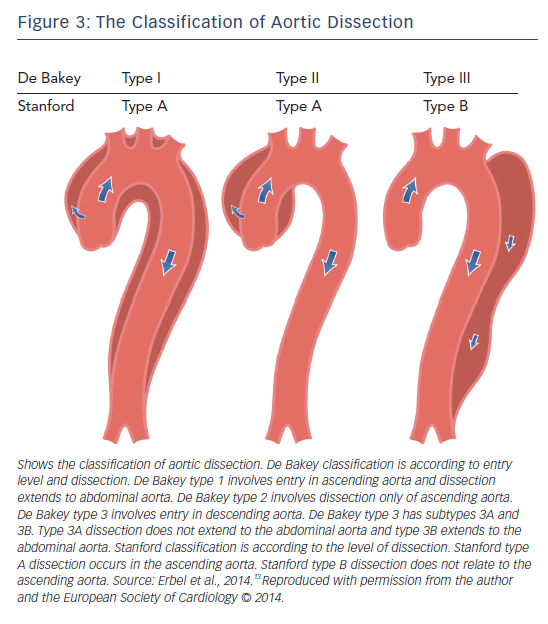
If a tear forms within the intima of the dilated aorta, aortic dissection may occur. Aortic dissection is defined as disruption of the medial aortic layer provoked by intramural bleeding, resulting in separation of the aortic wall layers and subsequent formation of a false lumen with or without communication with the true lumen.13 If dissection involves a coronary artery, myocardial infarction may also occur. Ischaemia of the mesenteric and femoral arteries has also been described in association with abdominal aortic dissection.10 The location of pain usually correlates with the location of the dissection; involvement of the ascending aorta causes anterior chest pain, of the descending aorta causes back pain and of the abdominal aorta causes abdominal pain. In MFS patients, dissection is most commonly Stanford type A involving the ascending aorta (see Figure 3).13 Aortic dilatation/dissection are major criteria for MFS diagnosis in the revised Ghent criteria, while MVP is included as a diagnostic systemic feature.18
Data from Januzzi et al.22 has demonstrated that 5 % of all patients with aortic dissection have a diagnosis of MFS. In comparing aortic dissection in patients with MFS and without MFS, type A dissection was more prevalent (76 versus 62 %; p=0.04) and intramural haematoma was less prevalent (2 versus 11 %; p=0.03) in MFS. Patients with aortic dissection and MFS were younger than those without MFS (35 ± 12 versus 64 ± 13; p<0.001), and less likely to have a history of hypertension (27 versus 74 %; p<0.001) or atherosclerosis (0 versus 32 %; p<0.001).12 In patients under forty years of age aortic dissection was less common (7 % of all patients with dissections), but a greater proportion of aortic dissections occurred in patients with MFS who were under forty.23 In the International Registry of Acute Aortic Dissection, 50 % of patients under 40 years with aortic dissection had MFS and 2 % of patients over 40 years with aortic dissection had MFS.23 Risk factors for aortic dissection in MFS are as follows: aortic diameter >5 cm, progressive aortic dilatation extending beyond the sinus of Valsalva, rapid aortic growth rate (>5 % per year or >2 mm per year in adults) and family history of aortic dissection.10
Sudden death from aortic dissection is decreasing due to improved aortic monitoring and elective aortic root surgery. However, after elective aortic root surgery, dilatation of the distal aorta is even more common,21 and careful monitoring of the entire aorta is important even though the aortic root has been repaired. Some patients will require surgery for distal aorta after aortic root surgery.20
In addition, pulse wave velocity (PWV) is higher in MFS patients and PWV of the whole aorta increases with age,24 indicating age-related aortic stiffening in MFS patients, with PWV a sensitive marker of aortic condition.
Aortic Valve
Aortic root dilatation causes aortic regurgitation (AR) with a central jet secondary to annular dilatation and/or myxomatous valvular degeneration.25 Patients usually have no symptoms during early stages. The severity of AR is evaluated by morphological features, Doppler index (including colour Doppler) and quantitative assessment using echocardiography. As the regurgitant volume increases, so does the volume of blood ejected with each LV contraction. Thus the end-diastolic LV volume becomes larger to maintain cardiac output, and the left atrium enlarges, reflecting the increased end-diastolic LV pressure and left atrial pressure. The LV ejection fraction does not decrease due to compensatory increases in ventricular contraction. Over time, chronic volume overload may result in myocardial damage and failure of the compensatory mechanisms such that cardiac output cannot be maintained. Myocardial damage affects the entire left ventricle and causes global hypokinesis.26
Mitral Valve
MFS patients have elongated and thickened mitral valve (MV) leaflets. This appears to be due to defective connective tissue and myxomatous degeneration secondary to increased TGF-beta signalling (see Figure 1).27 Ng et al.28 studied mice with FBN1 mutations to determine whether elongation and thickening of the MV leaflets was caused by increased activation and signalling of TGF-beta. They found that treating the mice with a TGF-beta neutralising antibody resulted in regression of the elongation and thickening of the leaflets, which returned to normal.
In the general population, the prevalence of isolated MVP is 2–3 %.29 In MFS the prevalence of MVP is 40 % and the prevalence of severe mitral regurgitation (MR) is 12 %.30 Anterior leaflet prolapse is more common than posterior leaflet or bileaflet prolapse, and prolapse of the posterior leaflet can predict severe MR.29 In contrast, another study reports that MVP is seen in 77 % of MFS patients carrying a FBN1 mutation.12
MVP carries a risk of developing MR, heart failure and infective endocarditis. Kühne et al. studied MFS patients with causative FBN1 mutations and moderate MR. Twenty-six percent of the group had progressive MR, associated with posterior leaflet prolapse and/or thickening, decreased LV ejection fraction, an increased indexed end-diastolic LV diameter, increased indexed left atrial diameter and tricuspid valve prolapse.31
When MR occurs, the left ventricle and left atrium become volume-overloaded, and heart failure may therefore occur in the decompensated phase.
Calcification of the MV annulus has been reported to occur at a greater rate in MFS than in normal individuals, and it is included in the diagnostic minor criteria in the cardiovascular system for MFS published in 1996.32 However, De Backer has studied some of the cardiovascular findings required in the criteria in 2009 and reported that calcification of MV annulus is not common nor practical for inclusion in MFS criteria.33
The severity of MR is variable. Some paediatric MFS patients have early onset and severe symptoms. In infancy, MR may cause congestive heart failure, pulmonary hypertension and death. More than 25 % of MFS patients have progressed from MVP to MR by adulthood, and twice as many women as men develop progressive mitral dysfunction.34
In 2011, Von Kodolitsch et al. reported that in MFS, the prevalence of MVP was 40 %, of severe MR was 12 % and of infective endocarditis was 2.5 %.29 More severe myxomatous MV thickening with prolapse occurred in 25 % of MFS patients.27 The reported outcomes of MVP and MVP-related events in 112 patients with MFS were as follows: bileaflet MVP affected 62 %, with anterior MVP occurring more often than posterior MVP. MR progression (related to a floppy MV and increased end-systolic LV dimensions) was seen in 37 %. MV infective endocarditis, heart failure and the need for MV replacement and repair were seen in 28 % (predicted by floppy MV and mild or moderate MR). Aortic dilatation, dural ectasia and ectopia lentis were not related to the outcome of MVP. Neither blood pressure nor medication (beta-blocker and angiotensin receptor blockers) had any effect on MV-related outcomes.29
Tricuspid Valve and the Pulmonary Artery
Tricuspid valve thickening, prolapse and regurgitation are common findings in MFS patients.1 These may occur due to degeneration of the tricuspid valve. In the study of Gu et al.,25 tricuspid valve involvement was seen in 12 % of MFS patients who underwent valvular or aortic surgery, severe tricuspid valve regurgitation was seen in 3 % of them and required valve repair. Moreover, tricuspid valve involvement sometimes occurred with MV involvement.
PA dilatation is also a minor criterion for MFS,32 usually occurring at the level of the root.35 In a cross-sectional study of adult MFS patients, computed tomography (CT) or magnetic resonance imaging (MRI) was performed to evaluate the PA.36 In normal subjects, the mean PA diameter was 24.0 ± 2.0 to 27.2 ± 3.0 mm. In MFS patients, the mean PA root diameter was 35 mm and mean PA trunk diameter was 29.8 mm. There was a correlation between PA dilatation and severity of ascending aortic disease. PA dilatation (>30 mm) was found in 54.0 % of patients with MFS, and 34.5 % of patients with MFS undergoing ascending aortic surgery. Both PA and trunk diameters were larger in patients who underwent ascending aortic surgery than in patients who did not (p=0.041).36 Another study published in 2014 also examined PA dilatation in MFS.37 It was reported that PA dilatation (upper normal limit set as 26 mm in this study) was seen significantly more often in MFS patients than in normal controls, 69.4 % and 4.9 %, respectively. They also reported that PA aneurysm existed in 15.3 % of MFS patients.37 Pulmonary hypertension can be the result.38,39
Left Ventricular Dilatation and Dysfunction
LV dimensions and configuration are normal in most MFS patients.40 A proportion (up to 7 %) have LV dilatation without other features of idiopathic dilated cardiomyopathy.40 In MFS patients with significant mitral, aortic or TR, LV dilatation and dysfunction may occur due to volume-overload. MVP may cause significant MR and heart failure, and 5 % of MFS patients with MVP develop heart failure.29 However, some MFS patients appear to develop ventricular dysfunction independently, in the absence of significant valvular regurgitation. Approximately 25 % of MFS patients have mild (<10 %), asymptomatic reduction in LV ejection fraction, suggesting impaired systolic function and underlying cardiomyopathy.41 In this group, 25.0 % have increased indexed left ventricular end-diastolic volume (LVEDV) and 30.9 % have increased left ventricular end-systolic volume (LVESV).41 Some of these patients also have right ventricular (RV) systolic dysfunction.41 LV ejection fraction and RV ejection fraction are strongly correlated.42 De Backer et al. reported some MFS patients have mild but significant LV systolic and diastolic dysfunction.43 To detect or predict changes of cardiac deformation or function, strain rate imaging may be useful for unoperated adults with MFS.44,45
Impaired LV ejection fraction is a key factor for sudden cardiac death (SCD).46 Although de Witte et al. reported that there were no correlations between aortic elasticity and LV ejection fraction,47 increases in LV afterload caused by aortic root stiffness48 as well as ECM remodelling and abnormal TGF-beta due to fibrillin deficiency, may contribute to impaired LV function.46 Afterload reducing agents can therefore improve cardiovascular function when congestive failure is present.48 It has been reported that 2.9 % of MFS patients without significant valvular disease experience heart failure.41 In MFS with end-stage heart failure, orthotopic transplantation is effective.49 In addition in MFS, acute onset heart failure may be caused by aortic dissection with aortic valve insufficiency.1
Arrhythmia and Sudden Cardiac Death
In 1997, Savolainen et al. investigated 45 adult patients with MFS50 and found the prevalence of cardiac arrhythmias, prolonged atrioventricular conduction, ST segment depression and ventricular repolarisation abnormalities such as QT interval prolongation, was higher than in the general population.50 Patients with MFS had more frequent atrial arrhythmias >1 extra premature beat/hour, ventricular arrhythmias >1 beat/hour, ventricular arrhythmias >10 beats/hour, ventricular salvos of >3 beats and R on T phenomenon. The most common arrhythmias were premature atrial or ventricular beats. This was not related to aortic root dilatation, left atrial dilatation, LV dilatation or function. It was presumed that deficiency of fibrillin-1 in patients with MFS, which causes microfibril abnormality in the matrix of the myocardium, affects conduction of impulses.50
Most arrhythmia does not lead to life threatening complications in patients with MFS; however, ventricular arrhythmia may occur in patients with repolarisation abnormality.50,51 Yetman et al. reported that ventricular arrhythmia was seen in 21 % of MFS patients and 4 % died from arrhythmia.51 Aydin et al.52 reported that in MFS, ventricular couplets and non-sustained ventricular tachycardia (VT) were seen in 40 % and premature ventricular contractions (PVCs) >10 beats/ hour were seen in 35 % of patients. PVCs, couplets, non-sustained VT and ventricular arrhythmias were related to N-terminal pro-brain natriuretic peptide (NT-proBNP) level elevation, left atrial dilatation, end-systolic LV dilatation, moderate MR and prolonged corrected QT (QTc). LV dysfunction and exon 24–32 mutations in the FBN1 gene also increased the risk of ventricular arrhythmia. In this cohort, the incidence of ventricular arrhythmias was 48 % and the incidence of ventricular arrhythmic event was 12 % including 4 % of SCD. The prevalence of ventricular arrhythmic events including SCD was 8 %, higher than in the general population.52 PVCs appear to be an independent risk factor for sudden death. Hoffmann et al. reported that 2.1 % of their MFS patients experienced SCD and 6.5 % had sustained VT. In this cohort, NT-proBNP levels ≥214.3 pg/ml carried a significant risk of SCD.46 These patients had a higher New York Heart Association (NYHA) classification and decreased LV function. Impaired LV function was also a risk factor for SCD. Overall the incidence of SCD in MFS is thought to be 0.92 per 1,000-person-years and in those with MFS aged 18–50 years is thought to be 0.09 per 1,000-personyears. Currently it is not possible to predict SCD by FBN1 mutation type.46 Abnormal heart rate turbulence parameters due to imbalanced autonomic dysfunction appears to be a risk factor for ventricular arrhythmia and SCD in patients with MFS.53
Investigations
Echocardiogram
Echocardiography is widely used for evaluation of cardiac valves and the aortic root, as it is convenient and non-invasive, and can be used in case of emergency. Following initial diagnosis, a second echocardiogram (ECG) should be performed after 6 months to assess the growth of aortic diameter and decide the interval for follow-up.54 Thereafter, echocardiography should be performed at least once a year in adults, unless aortic diameter is 4.5 cm or over, or there is recent major change in the aorta, when echocardiography should be performed twice a year.10 The incidence of aortic dissection is increased in those patients with more rapid increase in aortic diameter compared with those with a slower rate of growth.
Measurements of the aortic diameter are made in the parasternal long axis view at end-diastole using the leading-edge to leading-edge convention parallel to the aortic annular plane.55 In order to measure the maximum aortic diameter, the apical long axis view of the aorta is also helpful. Aortic diameter should be checked at the annulus, sinuses of Valsalva, ST junction, ascending aorta, descending aorta and abdominal aorta. The annulus is measured during mid-systole and other measurements are made at end-diastole.56
As mentioned before, aortic diameter depends on age, height and gender.19 Women have smaller aortas by 5 mm on average.57,58 Devereux et al. studied aortic root diameters in normal subjects and found that men had larger aortic diameters and so did people with a larger BSA.19 They devised the Z-score, calculated using height as shown below:19

In case of large BSA patients, it is suggested that this equation be used, as aortic diameter more than 40 mm should always be considered as dilated.56
Computed Tomography or Magnetic Resonance Imaging
CT, MRI and transoesophageal echocardiography all have high sensitivity and specificity for diagnosis of aortic dissection.10 CT is used for evaluation of the aorta and coronary arteries, and diagnosis of aortic dissection. MRI allows detailed assessment of the aorta, valve disease and ventricular size. If patients have chest deformities, the MRI is a good tool for evaluation. Evaluation of this systemic condition including whole aorta is possible by MRI without radiation. Two types of measurement of the aortic root are used: cusp– commissure and cusp–cusp. It is said that the aortic root diameter measured from cusp–commissure in diastole by non-contrast MRI is similar to the aortic diameter measured using inner edge–inner edge by echocardiography.59
For patients who have had surgery to replace their aortic root, CT or MRI should be done before discharge to evaluate structures beyond the aortic root, i.e. the aortic arch, descending aorta and the abdominal aorta.54 This should be repeated every 6 months until the aortic diameter has been stable, as some patients who have had aortic surgery will develop distal aortic dilatation.9
Electrocardiogram and 24-hour Electrocardiogram
The ECG in MFS sometimes demonstrates abnormal findings, including atrioventricular conduction delay, QT interval prolongation and ST depression. These findings do not appear to be related to aortic root diameter, but rather to cardiac structure and function and valve condition. As mentioned before, it is thought that fibrillin-1 deficiency causes microfibrillar abnormality in the myocardium, which in turn leads to impaired conduction.50 Repolarisation abnormalities are thought to be related to LV dilatation51 and PQ interval duration is thought to be associated with heart structure.50 The European Society of Cardiology guidelines suggest that patients who have cardiac symptoms should have a 24-hour ECG, as ventricular arrhythmia, conduction disorder and SCD may occur in this group.58
Prevention and Treatment
Daily Life
In the 1970s, the life expectancy of people with MFS was 40–50 years with death occurring due to aortic dissection, or heart failure from aortic or MR.60 Due to progression of medical and surgical therapy, life expectancy has vastly improved and now approaches that of the general population.61,62
MFS patients require appropriate exercise, isometric and isokinetic exertion at less than maximal effort. However, to avoid severe cardiovascular complications they should avoid contact sports, which may cause chest trauma, and also ocular complications such as ectopia lentis. Sports such as rugby and heavy weightlifting cause straining, which may produce Valsalva strain (breath-holding). Valsalva manoeuvres result in sudden increases in heart rate and blood pressure.63 Increasing intrathoracic pressure results in decreasing venous return, leading to systolic pressure and heart rate decrease. Due to baroreceptor reflex, heart rate and peripheral blood pressure increase. In general, patients with MFS are recommended to perform low- to moderate-intensity exercise. They should also have regular blood pressure checks and, according to European guidelines, blood pressure should be kept under 120/80 mmHg. If aortic dissection occurs, the target systolic blood pressure is lower still at 110 mmHg.58
Risk factors that accelerate expansion of the aortic wall should be addressed and modified. Smokers have a higher aortic dilatation expansion rate and higher occurrence of dissection than nonsmokers. Smoking should therefore be avoided by patients with MFS as it is related to vascular complications.64 About 30 % of patients with MFS have obstructive sleep apnoea (OSA) or central sleep apnoea (CSA).65 The reasons for OSA might be upper airway collapsibility, high nasal airway resistance and craniofacial abnormalities. There is an association of severity of sleep apnoea with aortic dilatation or dissection. It is thought to be related to progression of aortic dilatation, as this increases pleural pressure during sleep. As continuous positive airway pressure (CPAP) may stop aortic diameter progression, patients who have OSA should be treated with CPAP. Sleep apnoea is also an independent risk factor for reduced LV ejection fraction and increased NT-proBNP. Elevation of NT-proBNP is caused by both systolic and diastolic dysfunction. MFS patients with decreased ejection fraction have diastolic dysfunction before systolic dysfunction occurs.65,66
Medical Treatment
Beta-blockers
Beta-blockers are currently used to reduce the progression of aortic root dilatation. Frequency of aortic dissection and rupture is higher among hypertensive patients, and beta-blockers are therefore used to reduce these adverse aortic events in MFS. The target heart rate is under 60–70 beats per minute at rest and up to 100 beats per minute during exercise.10 Beta-blockers inhibit adrenergic betaactivation that occurs when adrenaline is released into the blood from sympathetic nerves and the adrenal gland. In the heart, betareceptor stimulation increases heart rate and cardiac contraction, and excitation conduction in the atrioventricular node. Thus beta-blocker therapy decreases heart rate, cardiac contraction and myocardial oxygen consumption. Some studies have shown that beta-blocker reduces blood pressure and heart rate, and thus reduces progression of aortic root size.67,68,69
Aortic stiffness is also reduced and aortic distensibility increased by beta-blockers.10 However, there are no reports which indicate that aortic dissection or prophylactic surgery can be completely avoided by beta-blockers.54
Beta-blockers are more effective in patients with less severe aortic dilatation, <4.0 cm.67 beta-blockers are recommended for use in the early stages regardless of aortic dilatation because the aorta dilates most between the ages of 6–14 years old.68 The main side effects of beta-blockers are bronchospasm, hypotension and bradycardia, and these should be monitored. Beta-blockers also affect glucose and lipid metabolism, and in patients with diabetes may mask the symptoms of hypoglycaemia, for example palpitations, tremor and hunger. In children, sleep disturbances are common side effects.70
Angiotensin Receptor Blocker
TGF-beta is produced when angiotensin II (AngII) combines with angiotensin II type 1 receptor (AT1R). As explained previously, TGF-beta signalling is excessive in MFS due to abnormal fibrillin-1. Increased activation and signalling of TGF-beta causes aneurysm formation and dissections due to ECM degradation, and consequent apoptosis and inflammation. As activation and signalling of TGF-beta are increased in MFS patients, inhibiting these pathways is felt to be effective therapy.7 Indeed, when mice with FBN1 mutations were treated with TGF-beta antibody, myxomatous degeneration of aortic and MVs was prevented. Use of AT1R antagonists (losartan) produced similar effects.71 Losartan is an angiotensin receptor blocker (ARB) used to control blood pressure. ARBs act on the renin-angiotensin pathway.72
The glomerulus in the kidney senses blood pressure. If blood pressure is low, renin is secreted, and if blood pressure is high, renin secretion decreases and renin concentrations affect those of angiotensinogen and angiotensin I (AngI). AngI is converted to AngII by angiotensin converting enzyme (ACE). AngII combines with the angiotensin II receptor; when it combines with AT1R in vascular smooth muscle, vasocontraction occurs, and when it combines with angiotensin II receptor type 2 (AT2R) in adrenal cortex, secretion of aldosterone is stimulated and causes increased blood pressure. ARBs inhibit AngII binding to AT1R, and ARBs reduce AngII action at the AT1R and AT2R. ARBs block AT2R producing antihypertensive effects and block the AT1R reducing endothelial production of TGF-beta. Additionally, AT2R inhibits AT1R, which inhibits ECM degradation, apoptosis and inflammation, thus delaying aneurysm formation.7,72
There are trials, some already reported and others ongoing, to evaluate the effect of losartan in MFS. According to a prospective, randomised controlled trial published in the Netherlands, losartan use reduced aortic root dilatation rate in adult patients with MFS.73 This study also showed that the reduction of aortic root dilatation rate was irrespective of age, sex, blood pressure, aortic root size, presence of FBN1 mutation and concomitant beta-blocker use. Another small trial in Taiwan also showed patients treated with losartan and beta-blocker demonstrated slower aortic dilatation progression than those on beta-blocker alone.74 However, these effects may not apply to children and adolescents. In 2014, Dietz et al. compared the effect of losartan versus atenolol over 3 years in 608 children and young adults with MFS who were 6 months to 25 years old. They found no significant difference in the rate of aortic root dilatation, aortic surgery, aortic dissection and death between the two regimens.75 Subsequently, a Spanish trial compared losartan versus placebo in 140 patients and aortic root progression rate was not different, although blood pressure was significantly decreased in the losartan group.76 Moreover, a French trial called Marfan Sartan also resulted in no significant difference in aortic root dilatation between the losartan and the placebo group.77 Considering these results, losartan might be effective for reducing the rate of aortic root dilatation and can be administered when patients are intolerant of beta-blocker; however, the effect of losartan reducing aortic root dilatation is not superior to that of beta-blocker. Hence, beta-blocker remains the first choice for treatment of MFS patients. Two further trials are ongoing in Italy and the UK at present. The Italian trial compares losartan versus nebivolol versus both, and the UK trial compares irbesartan versus placebo; these results are awaited.78,79
Angiotensin Convering Enzyme Inhibitors
Yetman et al. compared ACE inhibitor (enalapril) versus beta-blocker (atenolol or propranolol).80 Aortic distensibility was increased and aortic stiffness index was reduced in MFS patients. Aortic root diameter increase was equally likely in the two medication groups. ACE inhibitor reduces central arterial pressure and arterial stiffness.81 Activation of AT2R promotes apoptosis of vascular smooth muscle cells and cystic medial degeneration. ACE inhibitors can prevent cystic medial degeneration and apoptosis of vascular smooth muscle cells.82
However, if patients suffer from dry cough because of bradykinin-mediated side effects, taking this drug should be avoided.
Calcium Channel Blockers
Calcium channel blockers (CCBs) are sometimes used when the patient cannot take beta-blockers, for example because of asthma. However, no comparisons between CCBs and beta-blockers have been reported.83 One study comparing CCBs versus placebo in wild type mice and MFS mice was reported. According to this study, CCBs might increase the risk of aortic events such as aortic dissection or aortic surgery.84
Nitrates
Nitrates have the effect of venodilatation. These agents reduce pulse wave reflection from arterial branches, resulting in decreased central aortic pressure. This may decrease the rate of aortic dilatation progression and the tendency to dissect.83
Doxycycline
In 2008, it was shown that administration of the antibiotic doxycycline (a member of the tetracycline family) to mice, inhibited the expression of matrix metalloproteinase 2 and 9 (MMP-2 and -9) (both type 4 collagenases) and delayed elastic fibre disruption and aortic rupture.85 When doxycycline was compared with atenolol in the MFS animal model, animals administered doxycycline did not develop aortic aneurysms.86 Doxycycline is thought to affect endothelial function, elastic fibres86 and the structure of the aortic wall. However, as doxycycline has not been tested in patients with MFS, we do not yet have strong evidence to use this drug for inhibiting aortic dilatation.54
Management of Ventricular Arrhythmias
Patients with MFS should be carefully monitored for early detection of arrhythmia with resting and/or stress ECGs and periodic 24 hour ECG recordings.87 As arrhythmias may be secondary to other cardiac conditions, it is essential to also treat coexisting cardiac disorders. LV dilatation is associated with ventricular arrhythmias in MFS patients; this should be routinely measured and monitored through echocardiography. Beta-blockers, primarily used to prevent aortic dilatation, are thought to provide a parallel protection against arrhythmias. Sometimes atrial fibrillation and ventricular arrhythmia are related to the renin-angiotensin system (RAS), especially in the case of heart failure and myocardial hypertrophy. Agents like ARBs and ACE inhibitors are likely to play a role in reducing the incidence of arrhythmias indirectly by decreasing the primary cardiac disorder, and directly by modulating alterations in ion channels in RAS.87 However, further research is necessary to elucidate the potential role of RAS inhibitors in the prevention of cardiac arrhythmias in MFS patients. Hoffmann et al.46 demonstrated NT-proBNP to be an independent predictor of adverse arrhythmias in patients with MFS, and this new finding might help in selecting patients who are at risk of developing life threatening arrhythmias. Myocardial dysfunction may occur in patients with MFS and may lead to malignant arrhythmia such as ventricular fibrillation. In one case report, a MFS patient with impaired LV function received a cardiac resynchronisation therapy-defibrillator (CRT-D) and experienced improvement through reduced morbidity and less frequent hospitalisation for heart failure.88 Patients at high risk of developing malignant arrhythmias are referred for either an implantable cardiac defibrillator (ICD) or pacemaker.
Surgical Treatment
Prophylactic aortic replacement may be undertaken to avoid aortic dissection. European guidelines suggest that when the aortic root diameter reaches 5.0 cm, MFS patients should undergo surgery.58 At 4.5 cm, MFS patients are recommended to undergo surgery if they have risk factors for dissection, i.e. a family history of dissection, rate of growth >2 mm per year, severe AR or MR, or desire for pregnancy.58,89 However, as the routine cut-off measurement of 5.0 cm may be too late from a logistics perspective (due to constraints such as waiting time). A more realistic value of 4.8 cm is thought to be appropriate for referral. In cases of Stanford type A dissection, patients should be offered emergency surgery, because the mortality within a 48 hour period is 50 % (notwithstanding a perioperative mortality of 25 % and risk of complications).13 Surgery reduces 1 month mortality from 90 to 30 %, including dissection patients without MFS.13
Aortic root surgery is becoming safer. The mortality of elective surgery is 1.5 % compared to that of emergency surgery (11.7 %), survival rate at 5 years is 84 % and at 10 years is 75 %. However, MFS patients have a higher recurrence risk of dissection and aneurysm than in other aortic diseases.90 In Stanford type B dissection, which is seen in 10 % of all aortic dissection in patients with MFS,18 medical treatment is recommended unless there are complications requiring surgery. In all forms of acute aortic dissection, careful pain and blood pressure control is paramount.
Stent Graft
In aortic dissection type B with complications, for example ischaemia of the lower body or impending aortic rupture, surgery has a higher risk and a stent graft may be used to prevent false lumen enlargement. A stent graft may also be used for patients in whom open surgery is contraindicated.91,92 However, a systematic review reported that although MFS patients with dissection who have had stent graft had 1.9 % mortality, 21.6 % of patients had about 2–3 times the incidence of periprocedural endoleaks compared with non-MFS patients. The problem of endoleaks persisted at an average follow-up of 2.5 years and the final mortality was 12 %.93 For these reasons, stent graft for patients with MFS is not recommended.
Endocarditis
Infective endocarditis can be difficult to prevent as it may occur from such activities as brushing teeth, chewing or flossing.58 According to the National Institute for Health and Care Excellence (NICE) guidelines, patients with acquired valvular heart disease with regurgitation, previous infectious endocarditis or valve replacement are at risk of infectious endocarditis and are recommended prophylactic antibiotics.94 These guidelines do not recommend antibiotics for patients undergoing dental procedures, upper and lower gastrointestinal tract, genitourinary tract and upper and lower respiratory tract procedures.94 However, according to guidelines of the American Heart Association, some patients with MVP, surgical prosthetic material or a history of endocarditis should take prophylactic antibiotics when undergoing dental procedures or minor surgery.10.95
In the general population, endocarditis developed in only 0.48 % with asymptomatic MVP.96 On the other hand, in a cohort study, MVP and endocarditis were found in 40.0 % and 2.5 % of classic MFS patients, respectively,30 and it was reported that the chance of MFS patients developing MV endocarditis was 0.92 % at 30 years increasing to 13.42 % at 60 years of age.30 Another cohort study investigating classic MFS patients who had MVP with mild to moderate MR reported 6 % of patients developed MV endocarditis. MV-related events including MVP were supposed to be predicted by flail mitral leaflet and mild to moderate MR.29 MFS patients with MVP might have a higher risk of endocarditis.
The Marfan Foundation also recommends prophylaxis of endocarditis for MFS patients who have valvular regurgitation, because although other guidelines do not recommend prophylaxis of endocarditis for patients with valvular regurgitation without personal endocarditis history, there is no risk stratification of endocarditis in the case of patients with systemic connective tissue disorder.97
Conclusion
Treatment strategies for cardiac manifestations including betablockers, elective aortic root replacement and MV repair surgery have improved the life expectancy of people with MFS. Despite this improvement, cardiac morbidity remains a major concern among individuals with MFS. Newer approaches, such as the use of ARBs are believed to partly exert their beneficial effects by reducing TGFbeta activity, which has been recognised to play a pivotal role in the pathogenesis of the cardiac manifestations in MFS. However, ARBs have important limitations, and therefore further studies need to be performed to develop therapies that are specifically aimed at reducing TGF-beta activity.