Over the past 20 years, research and development in the field of oncology has produced significant changes and progress in cancer care. With the implementation of more aggressive cancer screening programmes, improvements in diagnostic testing and more effective treatment options, cancer death rates are gradually declining while cancer survivorship is steadily rising.1–3
Such statistics, however, have not been achieved without consequence, and are often offset by long-term adverse effects. While conventional chemotherapy has been known for decades to induce detrimental effects on the heart and peripheral vasculature, the use of novel agents are also increasingly being shown to have harmful off-target consequences to cardiac function. Thus, concurrent with advances in cancer therapies, so there has been a significant increase in cardiovascular side effects.4
One of the most common manifestations of cardiotoxicity associated with exposure to anticancer therapies is the development of left ventricular systolic dysfunction (LVSD) and overt heart failure (HF). As a result, the need for specialist cardiology input is becoming increasingly recognised as an important resource in the management of both long- term survivors and those undergoing active treatment. The aim of this paper is to review current opinions on the diagnosis, pathophysiology, management and prevention of chemotherapy-related cardiomyopathy, with specific focus on the commonest, and most studied culprits: the anthracyclines and monoclonal antibodies.
Definition of Chemotherapy-induced Cardiomyopathy
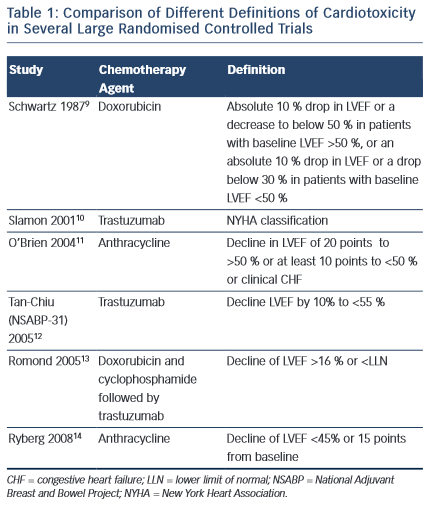
Despite the increasing recognition of chemotherapy-induced cardiomyopathy, consensus on international definitions in both clinical practice and trials remain lacking. Such definitions range from the development of HF symptoms, to the development of overt LV dysfunction and a reduction in ejection fraction (EF) on cardiac imaging (see Table 1). Indeed, the incidence of HF or LVSD in chemotherapy trials has been shown to range from 5 to 65 % depending on the criteria used.5,6 Moreover, it is widely accepted that chemotherapy- induced LVSD is often sub-clinical in the early stages, with overt changes in LVEF occurring after only a significant level of damage has occurred. Nevertheless, currently, a change in LVEF remains the basis for all definitions of cardiotoxicity issued by scientific societies in both Europe and the US.7,8
Anthracyclines
Anthracyclines are widely used to treat a variety of haematological, soft- tissue and solid malignancies. Cardiac toxicity has been recognised as a complication of treatment since the 1970s,15,16 with presentations ranging from subclinical ventricular dysfunction to severe cardiomyopathy and overt HF. Classically, cardiac dysfunction is related to anthracycline therapy in an exponentially dose-dependent manner. The early incidence of HF and LVSD ranges from 1 to 16 %, with increasing incidence as time post treatment progresses.17–19 Consequently, childhood cancer survivors have a high risk of experiencing symptomatic cardiac events at an early age, and this risk remains high for at least 30 years, when almost one in eight will experience severe heart disease.2
Pathophysiology of Anthracycline-related Cardiomyopathy
The cardiotoxic effects of the anthracyclines are not completely understood. Several mechanisms have been proposed, with the most widely accepted theory being the formation of anthracycline–iron complexes and stimulation of free-radical formation.2,20–23 In support of this is the finding that iron-chelating compounds inhibit this toxic effect.24 Despite this being the preferred theory, it is by no means the only mechanism by which the anthracyclines are thought to cause myocardial damage. More recently, Zhang et al.25 have demonstrated that, in mouse studies, deletion of the enzyme Top2b (encoding topoisomerase-II ) in cardiac myocytes was protective against the doxorubicin-induced DNA double-strand breaks and transcription changes that are responsible for defective mitochondrial biogenesis and the formation of reactive oxygen species. Furthermore, this deletion protected against the development of doxorubicin-induced HF, suggesting that doxorubicin-induced cardiotoxicity may be mediated by topoisomerase-II in mammalian cardiomyocytes.
Other proposed cardiotoxic actions of anthracyclines include: decreased adenosine triphosphate production; formation of toxic metabolites; inhibition of nucleic acid and protein synthesis; release of vasoactive amines; impairment of mitochondrial membrane binding, assembly and creatine kinase activity; induction of apoptosis; disturbances in intracellular calcium homeostasis; induction of nitric oxide synthetase; increased cytochrome C release from mitochondria; and increases in immune functions.14,26,27
Risk Factors for Anthracycline Related Cardiomyopathy
Perhaps the most predictive measure of the development of anthracycline-related cardiotoxicity is the total cumulative dose.28,29 In a review of three prospective trials assessing the effect of doxorubicin, Swain et al. demonstrated an incidence of HF of 3 % at a cumulative dose of 400 mg/m2, increasing to 7 % and 18 % at cumulative doses of 550 mg/m2 and 700 mg/m2, respectively.28 Combined with other treatment-related risk factors, such as additional cardiotoxic chemotherapeutic agents and radiotherapy, the incidence of HF is substantially increased.10
Several patient-related factors have also been identified as markers of risk in the anthracycline-treated population. These range from genetic predisposition (female sex, Trisomy 21, African-American ancestry,28,30–32 and carriers of the haemochromatosis C282Y HFE gene mutation33), to recognised pre-existing cardiac risk factors such as extremes of age, prior ischaemic heart disease, valvular heart disease and, in particular, hypertension.34
Prevention of Anthracycline-related Cardiomyopathy
A number of strategies and agents have been examined for potential use in preventing HF or LVSD related to anthracycline therapy, with varying levels of success.26,35–61 Such strategies include attempts to reduce peak plasma concentrations, prevention of iron-dependent free-radical formation52 and the use of evidence-based HF medications.
Limitation of Peak Plasma Concentrations
Several strategies have been examined to limit peak plasma concentrations, including the use of liposomal anthracycline preparations, novel synthetic anthracyclines and infusion versus bolus administration. Despite promising results in terms of maintained anti-cancer therapy,35–37 many strategies to limit peak plasma concentrations have either been limited by side effects44 or have not been associated with significant reductions in long-term risk of cardiomyopathy.45,46,50,51,62,63 Most promising results have been with the use of liposomal preparations, with rates of cardiotoxicity being significantly lower compared with conventional preparations.36,37,64
Iron Binding
Dexrazoxane binds intracellular iron and prevents iron-dependent free-radical formation.52 Although initial results led to the approval of its use to prevent long-term cardiotoxicity in patients receiving doxorubicin or epirubicin,53,54 subsequent clinical trials reported cases of secondary leukaemia in children and adults.55,56
Angiotensin Converting Enzyme Inhibitors, Beta-Blockers and Mineralocorticoid Receptor Antagonists
Angiotensin converting enzyme inhibitors (ACE-I) and beta (ß)-blocker therapy have both been shown to have protective effects against chemotherapy-induced HF or LVSD in both animal models and in adult patients with early toxicity.57–59 More recently, the results of the preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies (OVERCOME) trial60 have demonstrated that, compared with those in the treatment arm, those in the control group had a significantly higher reduction in LVEF, incidence of death or HF at six-months follow-up (p=0.02). Less evidence exists for the use of mineralocorticoid receptor antagonists (MRAs). In one recently published small randomised controlled trial, Akpek et al.61 demonstrated preservation of LV systolic and diastolic function in the group treated with spironolactone prior to the initiation of chemotherapy at 6-months follow-up. Further studies are needed to corroborate these findings.
Trastuzumab (Herceptin®)
Trastuzumab is a monoclonal antibody used in the treatment of HER2+ breast cancer. It exerts its actions by blocking the HER2 epidermal growth factor receptor, which is overexpressed in approximately 15–20 % of breast tumours and associated with more aggressive disease.65,66 Following phase III trials demonstrating significant improvements in both overall survival and risk of relapse, it was approved for clinical use in 1998.67
Introduction of trastuzumab into routine clinical practice, however, led to the unexpected finding of ‘off-target’ effects on the cardiovascular system. In one landmark trial, Slamon et al. demonstrated incidence rates of cardiac dysfunction of 27 % and New York Heart Association (NYHA) class III/IV symptoms of 16 % when trastuzumab was used in combination with both anthracyclines and cyclophosphamide.10
Pathophysiology of Trastuzumab-related Cardiomyopathy
The pathogenesis of trastuzumab cardiotoxicity is not completely understood, but appears to be related to blockage of HER2 receptor signals in cardiac myocytes – signalling that appears essential for cardiac myocyte repair.68
When given concurrently with anthracyclines, it has been proposed that the administration of anthracyclines results in initial oxidative damage to cardiac myocytes, resulting in those cells sustaining sufficient damage undergoing apoptosis or necrosis. The remaining injured myocytes undergo repair processes but remain temporarily vulnerable – the so-called ‘vulnerable window hypothesis’.69 In the presence of trastuzumab, inhibition of the normally upregulated HER2 results in loss of the usual repair mechanisms, driving the myocytes to further apoptosis and necrosis. In support of this theory are the findings of later studies examining the use of sequential anthracyclines and trastuzumab therapy. In these trials, initiation of a 3-week interval between treatments resulted in a reduction in the incidence of NYHA class III/IV HF to 3.8 %.12 With a longer 90-day interval, this reduced to just 0.6 %, with systolic dysfunction occurring in only 7 %70 compared with the 27 % reported by Slamon et al.10
In most instances, HF or LVSD related to trastuzumab is a sub-acute phenomenon, with the majority of cases being observed during treatment. It does, however, appear to be reversible after drug withdrawal and does not appear to be dose dependent.4
Risk Factors for Trastuzumab-related Cardiomyopathy
Apart from the well-recognised and documented risk in the setting of concurrent anthracycline therapy, risk factors for the development of trastuzumab-induced HF or LVSD are less well defined. In part, this is due to the exclusion of both older patients and those with ‘traditional’ risk factors for cardiac disease from clinical trials. Several retrospective cohort analyses have attempted to address this matter.68,71,72 Results suggest those with borderline lower limit of normal LVEF, history of heart disease or prior treatment with anti-hypertensive medication and those of advanced age have the highest risk of cardiotoxicity.
Prevention of Trastuzumab-related Cardiomyopathy
Few strategies have been adequately evaluated to determine their usefulness in preventing trastuzumab-induced HF or LVSD. Aside from delaying therapy until after treatment with anthracyclines is complete, possible strategies could include minimising trastuzumab treatment duration or the use of evidence-based HF medication.
Optimum duration for trastuzumab therapy remains unknown. Results from the Herceptin Adjuvant (HERA) trial comparing treatment over 12 and 24 months demonstrated no additional survival advantage with extended therapy, but did demonstrate continued risk of HF or LVSD.73 The Protocol of Herceptin Adjuvant with Reduced Exposure (PHARE) trial compared six versus 12 months of trastuzumab and failed to show non-inferiority of the shorter treatment administration but did demonstrate reduced incidence of cardiac events.74 Results from the Synergism or Long Duration (SOLD),75 Short-HER76 and PERSEPHONE77 trials are awaited to validate these findings.
No large randomised controlled trials have yet to report on the use of prognostically indicated HF medications to prevent HF or LVSD by trastuzumab. The Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research (MANTICORE) trial is due to address this issue by evaluating the efficacy of perindopril or bisoprolol for the prevention of LV remodelling in women with early breast cancer scheduled for chemotherapy and 1 year of trastuzumab.78
Other Chemotherapy Agents
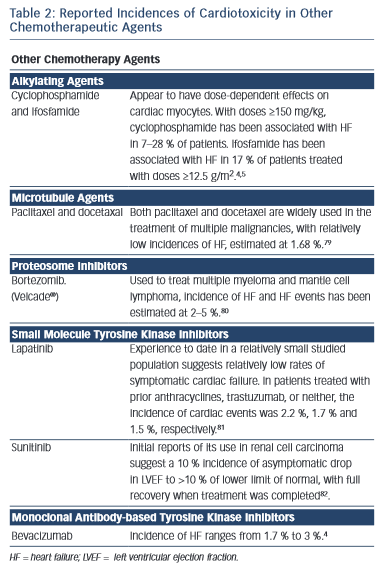
Several other chemotherapy agents have also been associated with the development of heart failure (see Table 2).4,11,79–82
Monitoring Cardiotoxicity of Chemotherapy
Cardiac Imaging
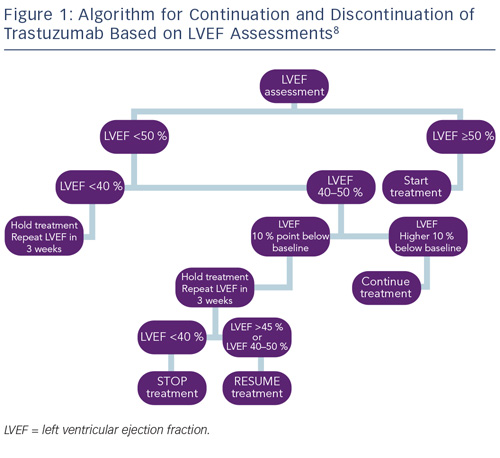
Monitoring cardiac function for early disease is recommended for all cancer survivors exposed to cardiotoxic therapies.8,83 As a result of the different timings of presentation and potential for reversibility, algorithms proposed by the European Society of Medical Oncology (ESMO) for monitoring patients exposed to anthracycline or trastuzumab have been developed to reflect these differences. Most notably, guidelines for trastuzumab incorporate advice on continuation or discontinuation of therapy and allow for the presence of potentially reversible changes (see Figure 1).
In recognition of the limitations of LVEF in detecting sub-clinical cardiotoxicity, several studies have examined the use of more sophisticated techniques, such as tissue velocity and strain imaging by echocardiography, and delayed contrast enhancement by cardiac magnetic resonance imaging (CMRI).
A recent meta-analysis of the use of myocardial strain imaging by echocardiography showed that, in late survivors of cancer, measures of global radial and circumferential strain are consistently abnormal, even in the context of normal LVEF.84 Their clinical value in predicting subsequent LVSD or HF, however, has yet to be fully explored, however, speckle tracking echocardiography (STE) and assessment of peak systolic global longitudinal strain (GLS) appears to be the most promising measure, with a 10–15 % early reduction in GLS by STE during therapy being predictive of cardiotoxicity.
Several studies have examined the use of CMRI and delayed contrast enhancement in the setting of chemotherapy-induced cardiotoxicity. While no specific changes have been identified in the anthracycline population, trastuzumab has been associated with mid-wall delayed enhancement of the lateral wall.85,86 Such findings have, however, only been reported in a small number of patients and further work is ongoing.
Biomarkers
Over the last two decades, data examining the use of biomarkers has demonstrated their measurement to be a more sensitive and specific tool for early identification; assessment and monitoring of chemotherapy-induced cardiac injury.69,87–89
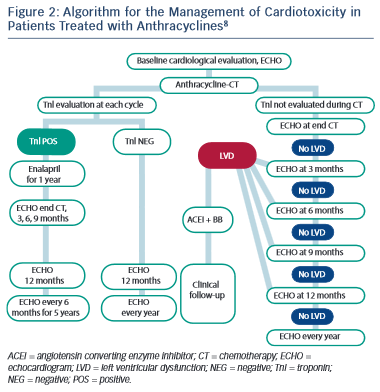
Several studies have reported on the use of cardiac troponins during anthracycline treatment and subsequent development of LV dysfunction.88,90–92 As a result, the latest guidance from ESMO has advocated the use of troponin in routine monitoring of patients undergoing anthracycline chemotherapy (see Figure 2).8 The use of troponins to identify patients at risk of trastuzumab-induced HF or LVSD is less well defined, but has been shown to identify those at risk of developing LVSD and, among them, those less likely to recover.69,93
A number of studies have looked at the use of the B-type natriuretic peptides, brain natriuretic peptide/N-terminal pro-brain natriuretic peptide (BNP and NTproBNP), in predicting LVSD in both anthracycline- and trastuzumab-induced cardiotoxicity, with mixed results.94–99 Consequently, current routine use of the natriuretic peptides for monitoring cardiotoxic effects of chemotherapy remains controversial.
Treatment of Chemotherapy-induced
Left Ventricular Systolic Dysfunction
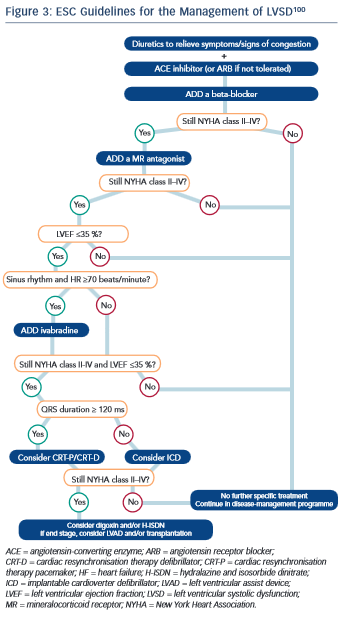
To date, no guidelines regarding the treatment of LVSD have been independently validated in the oncology setting. It is widely accepted, however, that once LVSD has ensued, treatment with evidence-based medications as per national and international guidelines should be instigated (see Figure 3).8,100,101
Of the evidence that does exist in the oncology setting, most has been based on treatment of anthracycline-related LVSD. In a study of 201 adult patients, Cardinale et al. demonstrated that LV dysfunction was potentially reversible but crucially depended on the time to treatment with ACE-I and ß-blockers, supporting the need for serial monitoring.102
In contrast to LVSD secondary to anthracyclines, LVSD induced by trastuzumab is less well studied, but the initial data are promising. In a small study by Ewer, et al.,103 38 patients with a diagnosis of trastuzumab-induced LVSD all patients demonstrated recovery of LV function. Thirty-two of these were temporally associated with medical therapy including ACE-I and ß-blockers, with the remainder recovering without treatment. Of particular interest, 25 of those recovering after medical therapy were subsequently re-challenged with trastuzumab, with only three experiencing recurrence of LVSD. These results require confirmation in larger, randomised controlled trials but do indicate that monitoring may be a viable option for some patients with trastuzumab- induced cardiotoxicity and re-challenge may be ‘safe’. Moreover, several studies have demonstrated that LVSD related to trastuzumab therapy appears to be reversible, with resolution of cardiac function in approximately 80 % of cases seen in the majority of trials.73 As a result of these findings, guidelines from ESMO suggest that the introduction of prognostically indicated HF medication may not be required unless the LVEF is <40 %.8
Conclusion
Chemotherapy-related cardiotoxicity in the form of HF or LVSD is a serious ‘off-target’ side effect of several important chemotherapeutic agents. At present monitoring of cardiac function and intervention in the presence of deterioration is the mainstay of management, but it is widely acknowledged that earlier detection and intervention is required to improve longer-term prognosis. Advanced imaging techniques and the use of cardiac specific biomarkers may herald significant changes in our management of this condition. It is inevitable, however, that with continued advances in the field of oncology, the incidence of ‘off-target’ cardiac side effects will likely increase. Cross-specialty collaboration will undoubtedly be the key to ensuring the best care for cancer patients.