Ischaemic heart disease (IHD) is the leading cause of morbidity and mortality worldwide. Two major pathways of disease development are acute coronary syndrome (ACS) and stable chronic angina.1,2 The success of prevention measures and early treatment pathways in ACS has helped to reduce the heart failure (HF) burden due to post-infarction remodelling and systolic dysfunction. However, accurately identifying patients with stable angina and relevant obstructive coronary artery disease (CAD) remains an on-going clinical challenge. The guidance on effective therapy for prognostic benefit is also continuously refined.3
In patients with chronic IHD, therapeutic strategies include medical management – which is a combination of risk-factor modification – or revascularisation in the presence of persistent symptoms and evidence of significant ischaemia.4 The need for revascularisation continues to be guided by way of pre-test probability (PTP) for CAD as a gatekeeper to the next diagnostic step and to evaluate the presence of relevant myocardial ischaemia.
If the PTP is high, an invasive strategy accompanied by measurement of fractional flow reserve (FFR) is advised.5,6 In the more prevalent group who have intermediate PTP, a non-invasive diagnostic test is first used. Then, if significant ischaemia is proven, the patient proceeds to coronary angiography and revascularisation of the target vessel. Patients with low PTP are deemed to not have any relevant ischaemia at all and other options of cardiovascular diseases are not routinely considered.
In the intermediate PTP group, a plethora of reasonably accurate non-invasive diagnostic tests exists. These include stress echocardiography, cardiac magnetic resonance (CMR) and nuclear imaging methods, single-photon emission CT (SPECT) and PET.2 Among these, the overall body of evidence – including validation, comparative diagnostic accuracy and, more recently the clinical effectiveness of directly-guided revascularisation – distinguishes CMR among the available imaging options.7 In addition to the many practical advantages, which include the absence of ionising radiation, non-invasiveness and accurate and reproducible measurements, it is the unique ability of ischaemia imaging and tissue characterisation that further separates this diagnostic approach. The three imaging readouts, myocardial perfusion, late gadolinium enhancement (LGE) and myocardial T1 mapping techniques, together allow a comprehensive assessment with diagnostic and prognostic relevance.8–11 In this article, we summarise the evidence behind these imaging readouts and outline the ways of supporting an informed individual approach to treatment of patients with stable chronic CAD.
Ischaemia Detection and Quantification
The primary utility of angiographical assessment of the severity and extent of epicardial coronary artery stenosis in stable CAD is based on historical evidence that significant (>70% lumen reduction) triple vessel disease and/or left main CAD lead to poor outcomes if left untreated.4 The many limitations regarding the visual or quantitative assessment of coronary artery anatomy, rather than haemodynamic significance, have been well covered elsewhere.6,12–14 The results of the Clinical Outcomes Utilizing Revascularization And Aggressive Drug Evaluation (COURAGE) trial indicated that revascularisation did not reduce the risk of major cardiovascular and cerebral adverse events (MACCE) in stable patients.15 The Fractional Flow Reserve Versus Angiography For Multivessel Evaluation (FAME) trials highlighted the need for an objective measurement of the significance of high-grade stenoses in terms of their functional relevance prior to intervention.6,16 While the superiority of an FFR-supported strategy in determination of haemodynamic significance corroborated the notion of previous observational imaging studies, it also opened up further unresolved questions for various non-invasive options of detection and quantification of myocardial ischaemia: which patients, which method and with what aim.16,17
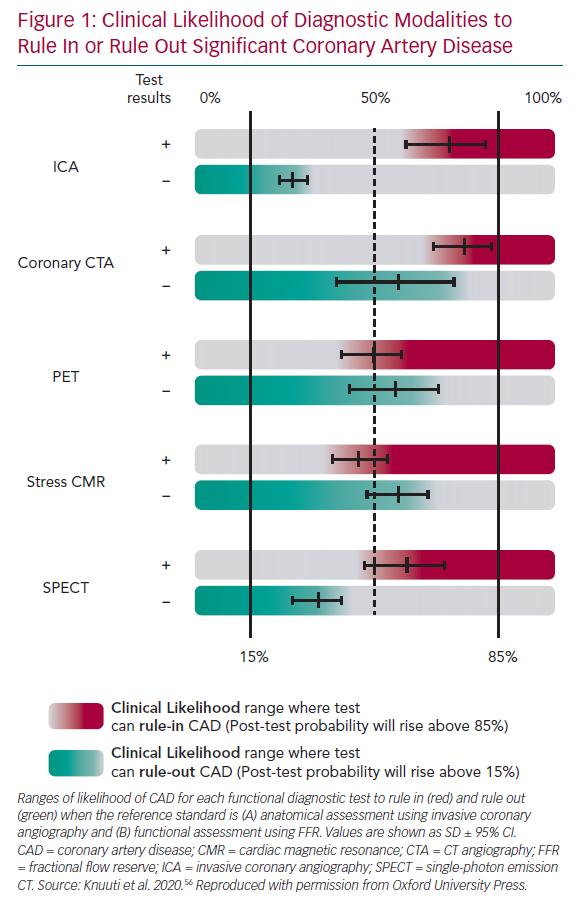
The choice of imaging approaches in guidelines – stress echo, SPECT, PET and perfusion CMR – remains guided primarily by the local availability of technique and expertise.2 Numerous studies have assessed their comparative diagnostic performance against clinical gold standards, angiographically determined luminal coronary stenosis and FFR, reporting good diagnostic accuracy for significant obstructive CAD (Figure 1). NB: data for stress-echo versus FFR are not available, and there are limited data for SPECT versus FFR.
The comprehensive body of evidence for CMR further strengthens its position, along with the available comparative data with SPECT, which reveals the superior diagnostic performance of CMR.17–19 This is also supported by data from meta-analyses.20,21 Furthermore, CMR can be distinguished from nuclear imaging methods in several practical ways, including the absence of exposure to ionising radiation and accurate multiparametric assessment (cardiac function, structure, flows and both scar and ischaemia imaging).
Shorter acquisition and post-processing times have cut the time and the costs of investigation and there is a much wider availability compared with PET. Although currently less used in favour of the simpler and safer vasodilatory test of myocardial perfusion, high dose dobutamine CMR yields an even higher diagnostic accuracy. CMR is also the only technique offering insight into comparative performance against dobutamine stress echocardiography.22 This prevailing data on the superiority of CMR (and PET) has now also been adopted in the European Society of Cardiology guidelines.2
Perfusion Techniques
Perfusion CMR imaging uses dynamic image acquisition of contrast agent wash-in into the myocardium using saturation-prepulse prepared T1 weighted gradient echo sequence. Saturation prepulse helps to blacken the background signal, allowing visualisation of the contrast-agent-induced brightness while washing into the myocardial muscle. A vasodilator substance – adenosine or the more specific A2A receptor agonist regadenoson – is typically used to stimulate blood flow through the myocardium. Although these medications are relatively safe, adenosine infusion is often accompanied with considerable chest discomfort.
These adverse effects are much milder with regadenoson, which is injected as a slow bolus mandating only one IV line. This allows much more amenable proceedings and an overall better image quality. During subsequent administration of gadolinium contrast agent, typically three short-axis images per cardiac cycle are obtained continuously and consecutively for about 60 heart beats to acquire the myocardial wash-in and wash-out (e.g. gadobutrol dose 0.05–0.1 mmol/kg bodyweight).7,23,24
In routine clinical practice, visual assessment is the mainstay approach to recognition of relevant hypoperfusion. Normal myocardium exhibits a characteristic homogenous distribution of rapid contrast uptake. The typical contrast agent wash-in begins at the epicardium of the left anterior descending coronary artery and then proceeds rapidly towards the endocardium as well as the right coronary artery territory.
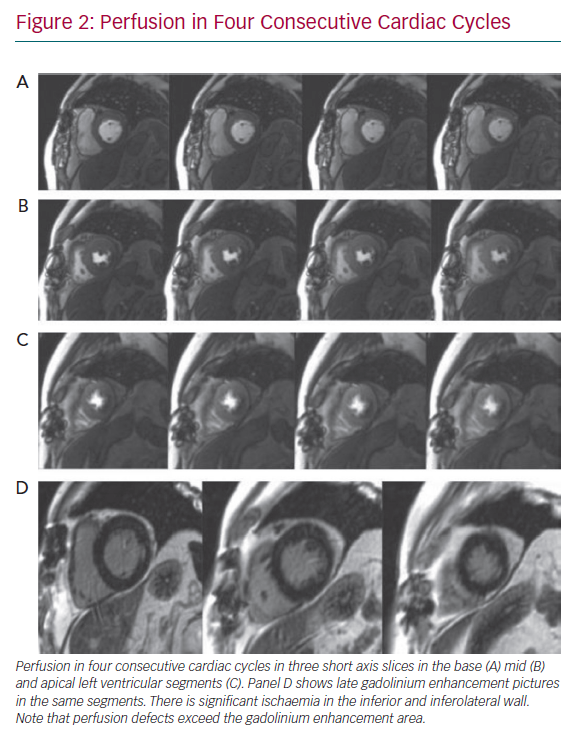
Perfusion defects are recognised as areas of visually perceived low signal indicating reduced contrast uptake (i.e. hypoperfusion), which follow a typical segmental distribution of an epicardial coronary vessel and persist for four or more consecutive cardiac cycles (Figure 2). Areas of hypoperfusion are compared against the LGE images. Where there is post-infarction scar, careful assessment is made to identify peri-infarct ischaemia. Quantitative approaches to myocardial blood flow and flow reserve assessment continue to be an active research area and have been well covered elsewhere.25 They may be particularly useful in assessment of intermediate stenoses given the on-going lack of clarity in defining its presence, as there is considerable discrepancy between the FFR and angiography, as well as an overall absence of outcome data on their prognostic relevance.17
Perfusion Cardiovascular Magnetic Resonance as a Tool to Guide Therapy
Functional proof of ischaemia remains the major criterion for prognostically relevant CAD.3,26 Furthermore, the extent of ischaemia (in addition to the presence of postinfarction scar and native T1 of non-infracted myocardium) is directly related to the number of subsequent CAD events. Primarily based on SPECT studies, the yearly CAD event rates generally range from approximately 1% for normal stress imaging findings to as high as 10% for severely abnormal studies. Several observational studies have also shown that the degree of relative risk reduction with treatment is related to the amount of ischaemia observed on non-invasive imaging.3,26 Yet the exact definition of ischaemic burden and thresholds for initiating revascularisation remains a subject of considerable debate, in part because of the difficulty in translating the proof and severity of ischaemia into comparable categories across other imaging modalities.3
Observational evidence indicates that medical therapy alone may be associated with a reduced risk of death compared with revascularisation for patients with less extensive and severe ischaemia (i.e. <10% of the myocardium). Conversely, patients with ≥10% ischaemic myocardium demonstrated a reduced risk of CAD and all-cause death with coronary revascularisation compared with medical therapy.26 Thus, the current practice guidelines support the requirement of moderate–severe ischaemia before elective revascularisation, whereby a threshold of ≥10% ischaemic myocardium provides a benchmark from which to define treatment effectiveness.26–28
Thresholds for relevant ischaemia using perfusion CMR follow this concept, with slight modification reflecting its technical advantages. 29,30 For example, the spatial resolution techniques of SPECT (10 mm × 10 mm
in-plane spatial resolution) only to detect transmural hypoperfusion, which can be recorded using a standard American Heart Association 16-segment model, meaning that relevant ischaemia will be defined as the presence of two adjacent hypoperfused segments. In contrast, higher spatial resolution of CMR allows recording of even smaller myocardial areas of hypoperfusion (with standard sequences of 3 mm × 3 mm in-plane resolution, with some advanced techniques perhaps also achieving 1 mm × 1 mm). Consequently, in CMR a 32-segment model is used, with division of each segment into endocardial and epicardial subsegments, also accounting for physiological distribution of contrast agent wash-in from epicardium into endocardium. In CMR, four or more affected subsegments are required to indicate relevant myocardial ischaemia (Figure 3). New data suggest that a further subdivision of segments may foster an even better diagnostic performance.31
The recently published MR Perfusion Imaging To Guide Management Of Patients With Stable Coronary Artery Disease (MR-INFORM) study is an international, multicentre, randomised controlled clinical effectiveness trial that has clarified several aspects of the therapeutic conundrum in patients with medium to high PTP for CAD.7 It investigated the ability of perfusion CMR to directly guide revascularisation compared with the standard of care based on FFR measurement. In the MRI-guided group, a positive CMR perfusion finding followed the above-mentioned definition, which was expanded for ease of interpretation to either two or more neighbouring segments, two adjacent slices, or a single transmural segment (approximately 6% of the myocardium). In the FFR arm, a measurement of ≤0.80 was defined as positive for relevant ischaemia in the target vessel. Revascularisation was recommended for patients in the CMR group with ischaemia in at least 6% of the myocardium or in the FFR group with an FFR of ≤0.8. Patients with a positive index test underwent revascularisation and all patients received guideline-directed medical therapy. The trial was designed to assess non-inferiority of the non-invasive ischaemic test to FFR in terms of 1-year outcome, defined by the composite primary outcome of death, nonfatal MI, or target-vessel revascularisation. The most important result was the non-significant difference between approaches in guiding treatment in terms of major adverse events (p=0.21). CMR was associated with a lower incidence of coronary revascularisation than FFR (36% versus 44.2%; p<0.01), which led to a considerable reduction in unnecessary invasive angiography to 51% of patients owing to having negative CMR test. These findings support perfusion CMR as the first-line approach in identifying patients who would benefit from treatment by revascularisation.
Chronic Coronary Artery Disease: Interstitial Myocardial Remodelling
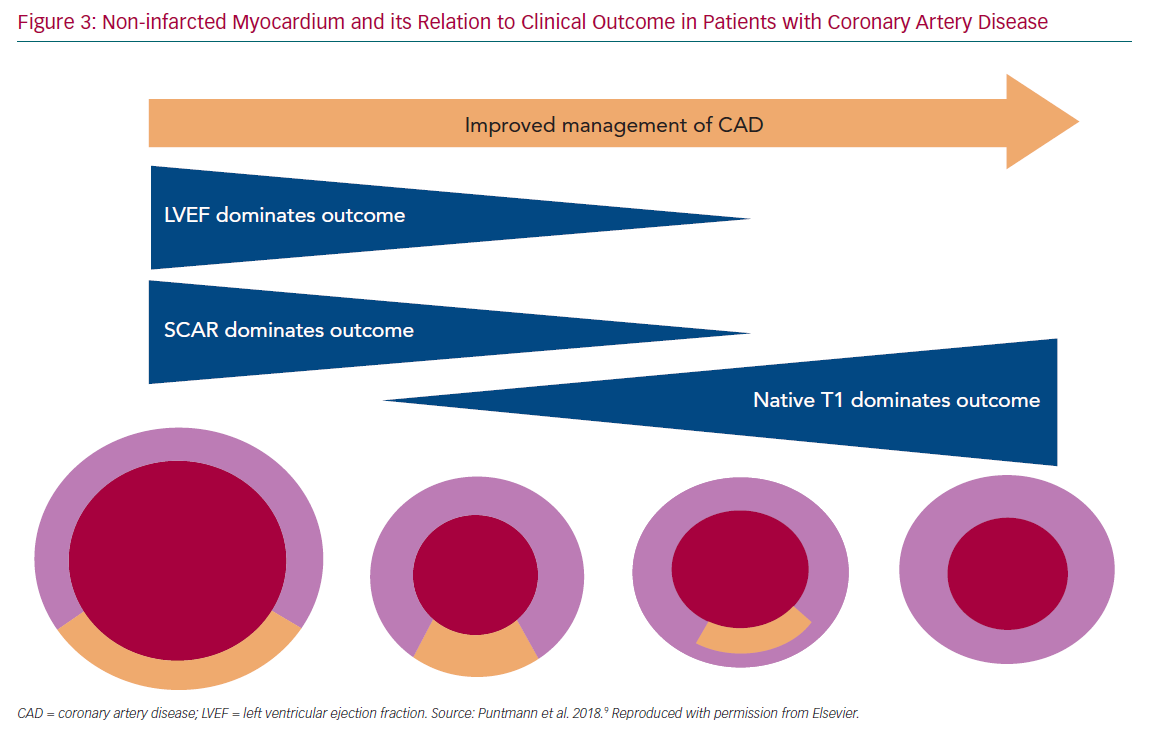
Diffuse interstitial myocardial fibrosis is the histological hallmark of myocardial remodelling, including in patients with CAD.32,33 Chronic neurohormonal stimulation and changes in gene expression promote the extracellular matrix remodelling processes in non-infarcted segments, eventually leading to accumulation of diffuse fibrosis.34 Although adaptive at first, this process becomes maladaptive, eventually leading to HF and poor prognosis.35–46 The considerable success of anti-remodelling therapies indicates that – despite the often late diagnosis – there is considerable potential for improvement prior to definitive or irreversible ventricular systolic dysfunction and abnormal remodelling with increased ventricular volumes and stiffness.32 Traditionally, low left ventricular ejection fraction (LVEF) has been the marker of poor outcome in post-MI patients, with LVEF ≤35% denoting high-risk and used as a surrogate for a more aggressive management.1 The extent of scar is also a strong predictor for adverse outcome.38,43–46
In modern populations of CVD patients receiving revascularisation therapies and strong prevention measures, the residual infarct size is relatively small and cardiac function is frequently preserved. With an overall reduced importance of the two traditional parameters, LVEF and LGE, the focus has shifted onto the non-infarcted myocardium. Studies have shown that T1 mapping indices, expressed by native T1 and extracellular volume and measured in the non-infarcted myocardium, increase in response to accumulation of diffuse myocardial fibrosis (Figure 3).38–41 These measures generally reflect the presence of the diffuse pathological processes that underlie myocardial remodelling, including inflammation, infiltration or fibrosis.41,42 Recent studies have demonstrated that T1 mapping measures are predictive of adverse outcome.9,47,48
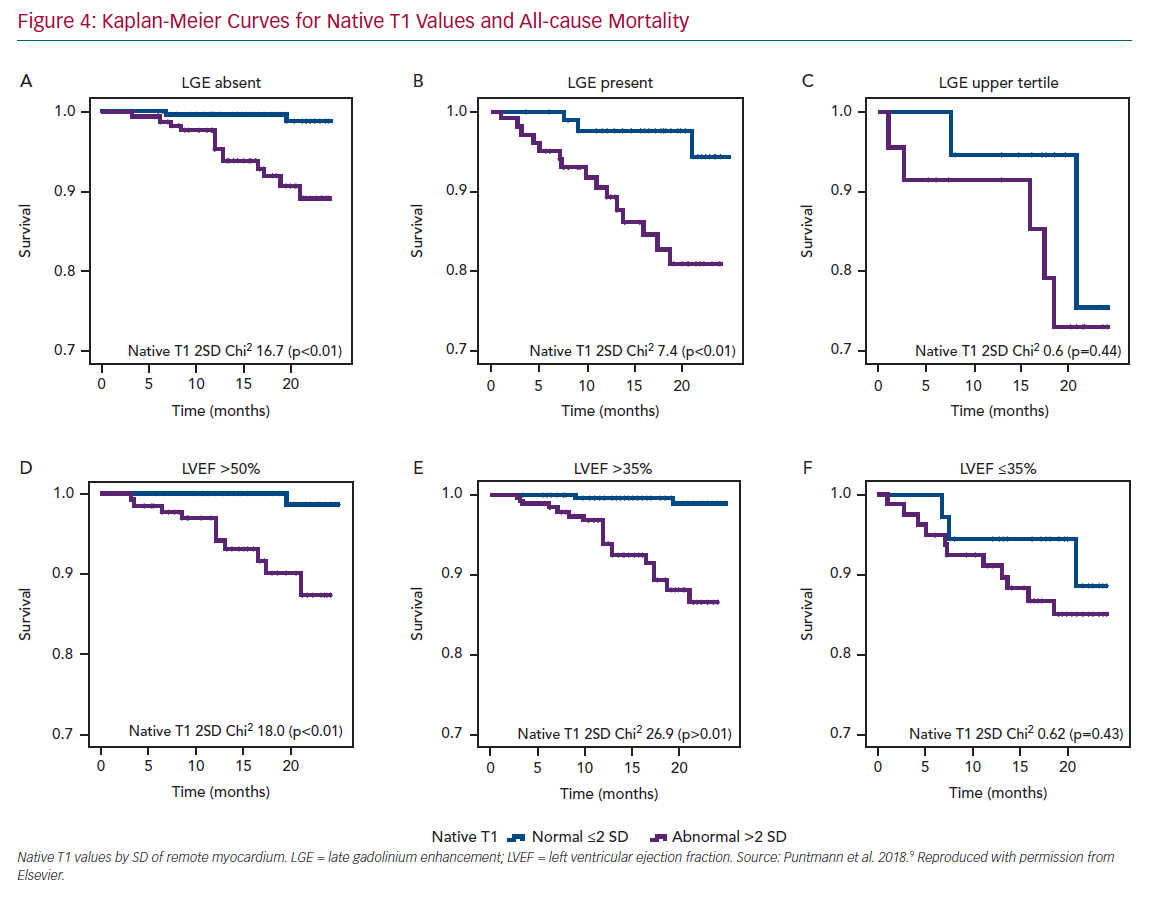
A single multicentre study in patients with chronic CAD revealed that native T1, the gadolinium-contrast free measure of non-infarcted myocardium was the strongest independent predictor of survival and major cardiocerebrovascular events.9 Moreover, with rising native T1 values, the likelihood of events was significantly increased. Patients with values in the upper tertile had 6.2-times greater likelihood of all-cause mortality and 4.5-times for MACCE, compared with those with native T1 within the normal range (Figure 4). However, the presence of LGE remained an important predictor of outcome only in those patients with considerably large scars and functional impairment. The findings of this study are important for several reasons.
Firstly, they are a testament to the importance of a direct measure of myocardial pathology, exposing the limitations of population-based risk scores that rely on indirect indicators such as cardiovascular risk factors. Native T1 reflects the presence and severity of myocardial changes and pathological myocardial remodelling, directly relating to the intrinsic myocardial disease such as the presence of myocardial oedema, inflammation, diffuse fibrosis and infiltration.49,51 These intrinsic disease mechanisms are, on one hand, pathophysiologically different and separate from the ischaemic injury as a result of MI, and on the other hand, central to prognosis in a prevalently scar-free CAD population. Secondly, native T1 is a quantitative biomarker; thus unsurprisingly, the prognosis is proportionally related to disease severity.
As a sensitive measure of pathological myocardial remodelling, native T1 may reflect a modifiable substrate and act as a potential therapeutic target, providing means of risk modification and improved prognosis. Sustained monitoring of native T1 levels may allow for an individual optimisation of treatment, possibly ahead of the symptom manifestation and development of phenotypically expressed, often an irreversible disease. Technical diversity in a field littered with various T1 mapping methodologies underlies the fact that the findings of the various studies are not transferable
owing to the different choices of T1 mapping sequences used.38,50 However, CMR with T1 mapping provides an important refinement of the current concept of risk assessment and may help to overcome an important gap in clinical management and discovery of therapies.51
Cardiovascular Magnetic Resonance and Health Socioeconomics
The cardiology practice guidelines set out the role of cardiac MRI as a part of clinical routine practice more firmly than ever before. Admittedly, the phrasing remains cautious: ‘a promising’ diagnostic tool with a ‘great potential’ to illuminate the underlying aetiology of heart disease. This rather reserved status of cardiac MRI continues to reflect an overall poor availability of CMR in routine practice and there are several aspects responsible for this.
Firstly, there is an on-going lack of scanner access, skill and expertise, in part a result of the perpetuating cardiology and radiology conflict over equipment sharing. Although there are international and all-inclusive CMR accreditation schemes, in most countries these are neither recognised nor integrated into traditional cardiology curriculum. Ironically, the absence of formal expertise remains the main argument of the national healthcare providers, precluding the rollout of the reimbursement in clinical practice.
Secondly, there is an overwhelming perception that the clinical market is not only saturated with MRI-based imaging, but that it is at risk of ‘overuse’, as overall morbidity and mortality has not been reduced despite a considerable increase in imaging over the last two decades. The caveat of this misconception is exposed by an organ-based breakdown of scanner utilisation, revealing that over 70% of MR scanner time worldwide goes to brain, spine and extremities, whereby cardiac MR imaging amounts to a mere 1%.52 Clearly, this deployment is illogically disproportionate to the magnitude of a problem created by heart disease, the major contributor to morbidity and mortality worldwide.
Finally, as imaging requires no regulatory evidence of clinical effectiveness in delivery of medical care (such as guiding treatment to change outcome), use is guided by a market of existing reimbursement schemes rather than a recognised clinical need. This also explains the persistent use of methods that are entitled to reimbursement despite recognised significant disadvantages and inferior evidence – such as nuclear medicine methods – and are simply underpowered to deliver the insights to a complex pathophysiology, for example, serial echocardiography, cardiac CT or invasive catheterisation.53–55 A move towards evidence-based use of imaging techniques in guiding treatment and improve outcome by way of regulation is pertinent to improve the current state of the art and stove off the rising HF epidemic, fuelled by the currently inadequate approaches.
Conclusion
The accurate diagnosis of underlying pathophysiology is paramount to safeguard good prognosis and the patient’s quality of life. Of the available diagnostic tools, CMR is distinguished by the overall body of evidence, ranging from validation, comparative diagnostic accuracy studies and, more recently, of clinical effectiveness of the direct guidance of revascularisation, showing that CMR is safe, delivering measurements that are accurate and highly reproducible, as well as provide valuable prognostic information. In addition to coronary artery disease and its complications, it is also able to elucidate the many non-ischaemic causes of symptoms in patients with relatively low pre-test probability for CAD.51 In survivors of acute MI, CMR can show the extent of salvages myocardium and, thus, the efficacy of treatment.52 This capacity renders CMR an invaluable modality for assessing the potential future advancements in treatment. Finally, CMR can illustrate the extent and severity of left ventricular remodelling following an MI, providing useful information for the management and prognosis of patients with ischaemic heart disease. This includes the transmurality of the scar, the degree of left ventricular dilatation, left ventricular ejection fraction and the extent of remote myocardial adverse remodelling. Despite being a powerful tool, the huge methodological diversity and lack of standardisation of CMR sequences and techniques poses an ongoing problem and a limitation to translation of scientific results into everyday practice, hindering collaboration among CMR centres and the understanding of disease pathophysiology.