Topic: 3. Heart Failure and Cardiomyopathy
Introduction and Objectives
LCZ696 has been considered class I B in the recent ESC Guidelines of heart failure (HF) for stable HF patients with left ventricular ejection fraction (LVEF) < 35 %, who remain symptomatic after the standard therapy, based on the results of the PARADIGM trial. This study showed a significant reduction in the risk of cardiovascular death and hospitalization for HF in patients treated with LCZ696 compared to those treated with enalapril. Although the study reported to improve the quality of life and NYHA functional class, it was not tested the functional capacity with any functional test. The 6-minute walk test (6MWT) is an easy method to objectively measure patients’ submaximal functional capacity because of its reproducibilIty, simplicity, and cost-effectiveness.
To analyse the impact of Sacubitril/Valsartan on functional capacity in patients with chronic HF.
Materials and Methods
Since October 2016, when the drug was marketed, all the patients referred to our HF-Unit who accomplished the 2016 HF ESC guidelines criteria for entresto were included in the study. A 6MWT was performed before starting the treatment and after three months of having optimized the dose. Physiological parameters and blood analysis were also measured at these time points.
Results
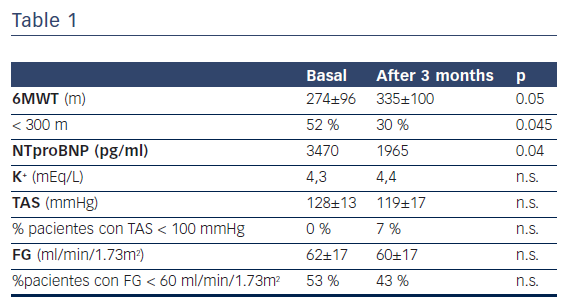
41 patients were screened and 30 of them entered in the study. These patients had the following characteristics: mean age 70, 80 % were males, mean LVEF was 30 %, isquemic etiology of HF 37 %, mean maximal tolerated dose 98mg/day. The results of the 6MWT, physiological parameters and blood samples are presented in Table1.
Results are mean or percentage. 6MWT: 6 minute walk test; M= meters; SBP: systolic blood pressure; GFR: glomerular filtration rate
Conclusions
Patients treated with Sacubitril/Valsartan improved their submaximal functional capacity at three months of treatment. Safety criteria such as hypotension or impaired renal function were not observed.