Aortic stenosis (AS) is a progressive disease of worsening left ventricular outflow obstruction. In the early stage of the disease, the prognosis is excellent with a very small risk of sudden death (<1 % per year).1–3 However, the development of a haemodynamically severe stenosis and the onset of symptoms herald a dramatic deterioration in prognosis. At this point, aortic valve replacement (AVR) provides symptomatic and survival benefits that generally outweigh the associated surgical risks.4–10
Echocardiography plays a critical role in the management of patients with suspected and documented AS by confirming the diagnosis, quantifying the haemodynamic severity and providing prognostic information important for predicting the clinical course and guiding management.9–11 Resting transthoracic echocardiography provides the mainstay technique for these purposes; however, transoesophageal and stress echocardiography can provide additional important information when encountering patients with discordant haemodynamic data, apparently asymptomatic haemodynamically severe AS and severe low-flow, low-gradient AS with or without a reduced left ventricular ejection fraction (LVEF). The purpose of this article is to review the echocardiographic assessment of the AS patient, highlighting important issues related to the assessment of haemodynamic severity, the prediction of clinical outcome and the use of echocardiography to guide patient management in both normal flow and low flow scenarios.
Criterion for Haemodynamically Severe Aortic Stenosis
Management of the AS patient is critically dependent on an accurate evaluation of the patient’s symptomatic status and the haemodynamic severity of the valve disease.9,10 Haemodynamic severity is used to decide whether symptoms are likely attributable to the valve and dictate the frequency of follow-up evaluations. Numerous haemodynamic indices have been proposed for this purpose; however, transvalvular velocity (VAS), transvalvular mean gradient (MG) and aortic valve area (AVA) derived by the continuity equation are the parameters recommended for routine clinical use due to their ability to predict clinical outcome.9–11 Haemodynamically severe AS has been defined by VAS≥4 m/s, MG≥40 mmHg and AVA≤1.0 cm2, because these findings are associated with a poor prognosis.9–11 The fulfilment of all three criteria essentially confirms the presence of severe AS; however, discordant findings occur in at least 30 % of AS patients with a normal LVEF, casting doubt about the disease severity.12–14 In patients with a reduced LVEF, discordant findings are even more common.15–19 In both groups, the VASt majority of the discordance relates to an AVA in the severe range, but MG or VAS in the non-severe range. In only a small minority of cases (<10 %), AVA is in the non-severe range, but MG or VAS is severe.12,14
Sorting Out Discrepant Haemodynamic Data – Aortic Valve Area is Severe, but the Mean Gradient is Not!
The Normal Left Ventricular Ejection Fraction Patient
Technical Reasons (Measurement Error)
Accurate measurement of the left ventricular outflow tract (LVOT) velocity, LVOT diameter and VAS are critical to the calculation of AVA using the continuity equation. Underestimation of the LVOT time velocity integral or diameter can lead to an erroneously small stroke volume (SV) and underestimation of AVA. Optimal LVOT velocity signals require the ultrasound beam to be aligned parallel to left ventricular (LV) outflow, the sample volume to be positioned immediately inferior to the aortic valve (5–10 mm) and the signal to show laminar flow with minimal spectral dispersion.11 The LVOT diameter measurement should be acquired on a zoomed parasternal long-axis image in mid-systole and measured 5 mm below cusp insertion.11 While measurements obtained at the annulus improve reproducibility, they tend to result in a larger AVA. The circular assumption used to derive the LVOT area from the diameter measurement is an important cause for underestimation of AVA as many patients have an elliptically shaped LVOT.20 The LVOT long-axis diameter often approximates the minor axis diameter and can lead to a 26 % underestimation of SV and AVA.21,22 Identification of the highest VAS is required to accurately measure MG and AVA, and Doppler interrogation of the aortic valve should be performed from the apical, right parasternal and suprasternal windows.23 Failure to identify the highest VAS will lead to an underestimation of MG, an overestimation of AVA and an underestimation of AS severity that tends to have a concordant rather than discordant MG and AVA.
When discrepant data are encountered, the initial approach should be to rule out measurement error. Echocardiographic images should be carefully reviewed for proper data acquisition and repeated if improper technique was used. The SV measurement should be confirmed by using another technique such as three-dimensional (3D)-echocardiography, or corroborating the continuity equation AVA by planimetry of the anatomic orifice using two-dimensional (2D) or preferably 3D-transoesophageal echocardiography.22,24 An AVA≤1.0 cm2 on planimetry confirms the presence of severe AS. However, an AVA slightly greater than 1.0 cm2 does not definitively exclude severe AS since the effective orifice area derived by continuity equation is usually slightly smaller than the anatomic AVA.
Patient Body Size
Cardiac output and AVA requirements are dependent on body size. In a small individual, an AVA≤1.0 cm2 may be relatively adequate for the cardiac output and generate a VAS<4 m/s or MG<40 mmHg. Conversely, an AVA>1.0 cm2 may be inadequate for a large individual, resulting in severe obstruction and a VAS≥4 m/s or MG≥40 mmHg. Current guidelines suggest that indexing AVA for body surface area (aortic valve area index [AVAi]) to account for different body sizes may be helpful and propose an AVAi≤0.6 cm2/m2 to identify severe AS.9–11 However, prognostic studies supporting this approach are limited.25 Indexing AVA to body surface area increases the prevalence of severe AS, but also increases the prevalence of discrepant haemodynamic data.12,14,26,27 In the SimVAStatin and Ezetimibe in Aortic Stenosis (SEAS) trial, AVAi did not improve the prediction of aortic valve events or cardioVAScular death compared to AVA.26,27 A linear relationship between AVA and body surface area may not be the optimal correction factor and likely oversimplifies the different implications of body size, overweight status and obesity that might require different correction factors.14,28 Nevertheless, body size should be considered when confronted with discordant haemodynamic data.
Inconsistencies Between Different Criteria for Severe Aortic Stenosis in the Valve Guidelines
Inherent in the definition of severe AS is that a MG of 40 mmHg equates to an AVA of 1.0 cm2. However, the relationship between MG and AVA is a function of cardiac output, heart rate and systolic ejection period. A patient with a cardiac output of 6 l/min, a heart rate of 70 bpm and a systolic ejection period of 330 msec would have a MG of 34 mmHg if AVA was 1.0 cm2, not 40 mmHg as suggested by the guidelines. Potentially, MG could be as low as 26 mmHg with an AVA of 1.0 cm2 despite a normal cardiac output.29
Low Flow State
MG is directly proportional to the squared function of transvalvular flow and can be small (<40 mmHg) despite an AVA≤1.0 cm2 if transvalvular flow is significantly reduced.16,18,19 Low flow is usually considered when the LVEF is reduced, but should not be excluded when the LVEF is normal. Low flow has been defined as an indexed stroke volume (SVi)<35 ml/m2;19,30 however, measures that account for the systolic ejection period (i.e. mean transvalvular flow rate <200–250 ml/s) are theoretically likely more appropriate. Hypertension, significant mitral or tricuspid valve disease, pulmonary artery hypertension and atrial fibrillation are clinical entities often co-existing with AS and associated with low flow despite a normal LVEF. Elevated blood pressure can reduce transvalvular flow and result in a smaller MG, smaller AVA and discordant measurements.31 In patients with uncontrolled hypertension, low flow and discordant data, blood pressure should be corrected and the echocardiogram repeated as normalisation of flow may resolve the discrepancy.
Low transvalvular flow can also be present in AS patients without concomitant valve disease or pulmonary artery hypertension, a clinical entity often referred to as paradoxical low flow AS, because of the paradoxical association of a low flow state despite a preserved LVEF.30 These patients tend to be older, female and have associated hypertension, metabolic syndrome, diabetes or atrial fibrillation.19,30,32–34 They also tend to have smaller left ventricular cavities with concentric remodeling, myocardial fibrosis, reduced compliance and restrictive physiology.19,34,35 Despite the preserved LVEF, they have evidence of intrinsic systolic dysfunction with impaired longitudinal shortening.34–37
The Reduced Left Ventricular Ejection Fraction Patient
The presence of a low flow state is often the first assumption when encountering discrepant haemodynamic data in the AS patient with a reduced LVEF. However, not all patients with a reduced LVEF have a low flow state. SVi or mean transvalvular flow rate (<35 ml/m2 and <200–250 ml/s, respectively) should be calculated to confirm the presence of low flow and measurement error excluded.
Additional Complementary Investigations
Discordant measurements can usually be resolved by systematically considering the above issues. However, multi-detector computed tomography (MDCT), magnetic resonance imaging (MRI) and cardiac catheterisation can be helpful when the cause for the discrepancy is not apparent and the patient has symptoms or unexplained left ventricular dysfunction. AVA, LVOT area and SV can be obtained non-inVASively using MDCT or MRI and compared with the echocardiography data.22,38–40 Aortic valve calcification (AVC) can be quantified using MDCT and may help clarify discordant data. AVC>~1,250 Agatston units (AU) in women and >~2,050 AU in men suggests the presence of severe AS.41 Cardiac catheterisation provides a direct measurement of MG or AVA by Gorlin equation, and can evaluate for coronary artery disease as an alternate explanation for symptoms or left ventricular dysfunction.39
Risk Stratification and the Prediction of Outcome Using Echocardiography
Asymptomatic Aortic Stenosis with Normal Left Ventricular Ejection Fraction
Peak Aortic Stenosis Velocity and the Rate of Haemodynamic Progression
VAS is the strongest predictor of clinical outcome, even in the subgroup with severe AS.1,2,42–45 For asymptomatic AS patients with VAS<3m/s, 3–4 m/s and >4 m/s, approximately 15 %, 35 % and 75 % of patients, respectively, will develop symptoms requiring AVR within three years.1–3,42,45 VAS>5.5 m/s is associated with a poor three year event-free survival of only 11 %,44 and the European Society of Cardiology (ESC) and American Heart Association (AHA)/ American College of Cardiology (ACC) valve guidelines consider AVR reasonable in these asymptomatic patients if the surgical risk is low (IIa recommendation).9,10 AVA also predicts event-free survival, although the relationship is not as robust as VAS.1,42,43,46 This may relate in part to an inherent bias by which clinicians may be more likely to refer patients with a higher VAS or MG for AVR. Rapid haemodynamic progression, defined as an increase in VAS≥0.3 m/s/yr, identifies patients at increased risk for clinical events.1,3,45 Severe AS patients with rapid haemodynamic progression have an event rate as high as 80 % in two years,3 and according to the ESC and AHA/ACC valve guidelines, AVR may be considered in an asymptomatic patient at low surgical risk (IIa and IIb recommendation, respectively).9,10
Valve Calcification
Valve calcification is best evaluated clinically using MDCT. However, valve calcification assessed by echocardiography is a strong predictor of haemodynamic and clinical progression across the spectrum of disease severity, and independent of the haemodynamic severity.3,45 Calcification can be graded as none, mild (isolated small spots), moderate (multiple bigger spots) and severe (extensive thickening/calcification of all cusps). Severe AS patients with moderate or severe valve calcification have an event rate of 80 % at four years, but only 25 % with no or mild calcification.3
Flow Pressure Gradient Classification
Patients with AVA≤1.0 cm2 and a normal LVEF can be classified into four subtypes based on the presence of:
- normal flow (NF) or low flow (LF) (SVi≥ or <35 ml/m2); and
- high MG (HG) or low MG (LG) (MG≥ or <40 mmHg).36,37
Most patients (52–73 %) have the NF/HG pattern, in which MG and AVA are concordant for severe AS and SVi is normal.30,32,36,37 The NF/ LG pattern is present in 15–31 %, and encompasses patients with inherent inconsistencies in the cut-points for severe AS, small body size and prolonged ejection times in which SVi is normal but transvalvular flow rate may be reduced. The LF/HG pattern is observed in 3–13 %, and includes patients with concordant indices but intrinsic left ventricular dysfunction despite a normal LVEF. The LF/LG pattern is present in 3–19 % of patients, a group characterised by discordant haemodynamic indices and a low flow state.
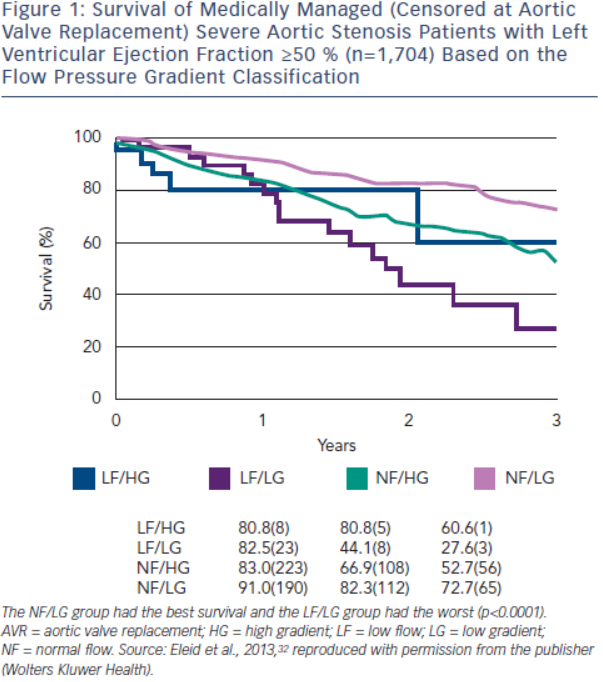
The flow pressure gradient classification provides important prognostic information for severe AS patients with a normal LVEF. The NF/LG pattern has the highest event-free survival, while the LF/LG pattern has the worst outcome.32,34,37,47 The NF/HG and LF/HG patterns have intermediate survival. In Eleid et al.,32 survival with medical management (censored at AVR) at two years was 82 % in NF/LG patients, 81 % in LF/HG patients, 67 % in NF/HG patients and 44 % in LF/LG patients (p<0.0001) (see Figure 1). AVR conferred a strong survival benefit in LF/LG and NF/HG patients, but no benefit in NF/LG patients. However, other investigators have observed a survival benefit of AVR in both symptomatic LF/LG and NF/LG patients.48–51 SVi appears to be an important marker of prognosis and has a graded association with mortality in severe low gradient AS patients with preserved LVEF.52,53
Left Ventricular Function
Only a minority of severe AS patients present with a reduced LVEF and the prognosis is poor without AVR.9,54 However, a large proportion of severe AS patients with normal LVEF have intrinsic systolic dysfunction with abnormal global longitudinal strain on speckle tracking imaging.55–57 Patients with moderate or severe AS and global longitudinal strain ≤15 % have a poor two-year event-free survival of ≈30 %, as compared with 65 % if >15 %.56,57 There are no data to justify AVR in asymptomatic patients with impaired longitudinal strain, although close follow-up is likely warranted.
Valvular Arterial Impedance
Valvular arterial impedance (Zva) provides a measure of global LV load due to the valve stenosis and VAScular afterload. Zva is calculated as (SBP+MG)/SVi, where SBP is the systolic blood pressure.58 In asymptomatic patients with moderate or severe AS, Zva>4.5–5.0 mmHg/ml/m2 predicts reduced event-free survival and a 2.8-fold increased risk of death;56,58 however, Zva does not identify the relative contributions of the valve and VASculature that is necessary to guide management (i.e. AVR versus blood pressure [BP] control).
Exercise Echocardiography
More than 50 % of patients with asymptomatic severe AS develop symptoms within three years, and the subsequent prognosis is poor without timely AVR.1–3,8 Exercise testing can unmask symptoms in as many as one third of patients and a positive exercise test predicts the need for AVR or death.59–61 The ESC and AHA/ACC valve guidelines consider AVR indicated in asymptomatic severe AS patients with symptoms on exercise testing (Class I recommendation) or a drop in BP (IIa recommendation).9,10
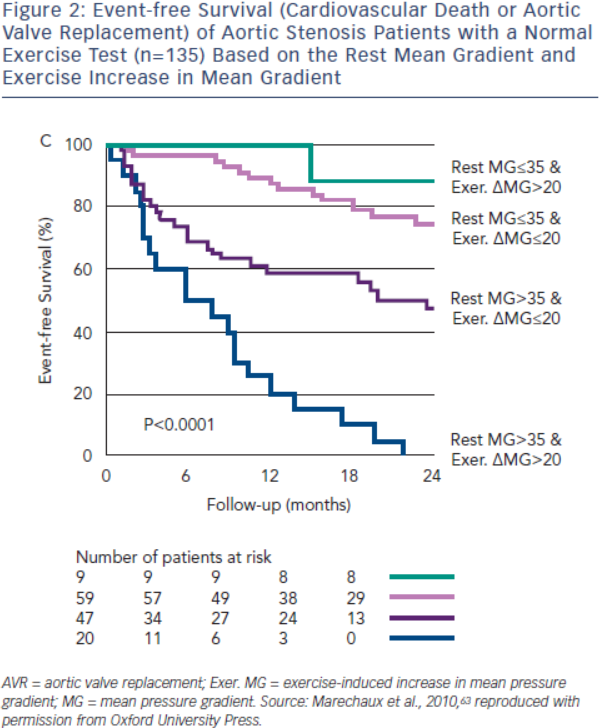
Exercise echocardiography may provide additional prognostic information beyond the exercise test. An exercise increase in MG>18–20 mmHg potentially identifies severe AS patients at increased risk for symptoms, AVR or death, even with a normal exercise test (see Figure 2).62,63 The adverse prognosis of patients with greater increases in MG likely reflects a more severely stenotic or non-compliant valve with minimal opening reserve. In the ESC guidelines, AVR may be considered in these patients if the surgical risk is low (IIb recommendation).10 Failure of LVEF to increase with exercise is also associated with an abnormal exercise test and reduced event-free survival (event-free survival <40 % at two years).64 Although not specifically addressed in either valve guidelines, these patients should at a minimum be observed closely for surgical indications. Resting and exercise changes in global longitudinal strain are associated with an abnormal stress test and reduced event-free survival; however, their incremental prognostic value requires further study.55,65
Pulmonary hypertension is associated with poor outcomes and higher complication rates with valve intervention.66,67 The prevalence of pulmonary hypertension at rest in asymptomatic AS patients is low; however, exercise-induced pulmonary hypertension may develop in >50 % of severe AS patients.68 Pulmonary systolic pressure >60 mmHg with exercise is associated with a twofold increased risk of cardiac events and a reduced cardiac event-free survival at three years (22 ± 7 versus 55 ± 9 %; p=0.014), independent of the change in MG with exercise.68 Exercise pulmonary hypertension appears to unmask a severe impairment of diastolic function. Close follow-up of these patients is likely warranted.
Low-flow, Low-gradient Aortic Stenosis
Low-flow, Low-gradient Severe Aortic Stenosis with Reduced Left Ventricular Ejection Fraction
Definition
Low-flow, low-gradient severe AS with reduced LVEF, or classical low-flow, low-gradient AS,19 is defined by a MG<40 mmHg or VAS<4 m/s, AVA≤1.0 cm2, and a LVEF<40–50 %.9–11 Inherent in the definition is that flow is reduced, although no specific criteria are included in the guidelines. Individual studies have used a cardiac index <3.0 l/min/m2 or SVi <35 ml/m2,18,19 although measures such as transvalvular flow rate (<200–250 ml/s) may be more appropriate. These patients represent a diagnostic and management challenge since the severity of the underlying valve stenosis is often unclear, AVR is associated with a high operative risk and the benefits of AVR to alleviate symptoms or significantly improve prognosis may be limited in some patients.16–19,54,69–71
True Versus Pseudo-severe Aortic Stenosis
AVA, whether measured by continuity equation or orifice planimetry, is affected by flow and becomes smaller when transvalvular flow is reduced.72–74 This phenomenon has been attributed to flow dependence of the flow convergence,75–77 flow-mediated effects on the vena contracta spatial velocity profile,76 reductions in anatomic orifice area due to smaller flow-mediated forces,78,79 or a combination of these factors. The potential effect is that AVA may appear only mild or moderately stenotic when assessed under normal flow conditions, but potentially severe when assessed under low flow conditions. The challenge when encountering the patient with low-flow, low-gradient severe AS with reduced LVEF is to distinguish the patient with ‘true’ severe AS (TSAS), where the valve is severely stenotic and afterload mismatch exists, from the patient with ‘pseudo-severe’ AS (PSAS), where the underlying valve is only mild or moderately stenotic, but appears severe due to low flow and the inability of a poorly functioning ventricle to sufficiently open the valve. AVR is likely beneficial in the TSAS patient, but may not be beneficial in the PSAS patient since the valve is not the primary problem. PSAS is reported to be present in 5–35 % of these patients depending on the specific diagnostic criteria used.69,70,80–82
TSAS and PSAS can be distinguished by augmenting transvalvular flow using dobutamine stress echocardiography (DSE) and re-evaluating the valve haemodynamics at a normal flow state. A commonly used DSE protocol employs an initial infusion of 2.5–5.0 ug/kg/min, followed by incremental infusions of 2.5–5.0 ug/kg/min at 5–8 minute stages to a maximum dose of 20 ug/kg/min.71,82 This low dose, longer stage protocol minimises the potential for myocardial ischaemia and allows for steady state acquisition of LVOT and transvalvular velocities. Patients with TSAS and an underlying ‘fixed’ valve stenosis would be expected to have little change in AVA and a large increase in MG during DSE. In contrast, patients with PSAS would be expected to have an increase in AVA outside of the severe range and only a small increase in MG due to the larger AVA.
Criteria used to diagnose TSAS have included a DSE peak MG>30–40 mmHg, an AVA increase <0.3 cm2, and/or a DSE peak AVA≤1.0–1.2 cm2.18,19,69,70,80–82 PSAS is suggested if the DSE peak AVA is >1.0–1.2 cm2 and the peak MG is ≤30 mmHg or the AVA increase is ≥0.3 cm2. In the AHA/ACC valve guidelines, AVR is considered reasonable in symptomatic patients with low-flow, low-gradient severe AS with reduced LVEF if VAS is ≥4.0 m/s or MG is ≥40 mmHg with an AVA≤1.0 cm2 at any time during DSE (IIa recommendation).9 While this criteria is specific for TSAS, many patients with TSAS have a MG<40 mmHg during DSE because of poor contractile reserve or inherent inconsistencies in the criteria for severe AS.69,82,83 A lower cut-point for MG (i.e. >30 mmHg) may be justified to avoid underdiagnosing TSAS patients in whom the prognosis without AVR is extremely poor.69–71,82,83
A significant limitation to distinguishing TSAS and PSAS patients relates to the marked inter-individual variability to which flow augmentation occurs with DSE.74,82 Some patients have very little increase in transvalvular flow during DSE and a low flow state persists. Other patients have a large increase in transvalvular flow with supranormal flow rates. Since the increase in MG and AVA are largely dependent on the magnitude of flow augmentation, interpretation of the underlying AS severity without consideration of the transvalvular flow is problematic. To address this issue, the projected AVA (AVAproj) at 250 ml/s has been proposed to standardise the calculation of AVA at a normal flow rate and better distinguish TSAS and PSAS.82,83 Transvalvular flow rate and AVA generally display a linear relationship in AS patients with the slope varying between patients.72,74 AVAproj can be calculated by measuring AVA and transvalvular flow rate at a minimum of two different flow rates during DSE using the equation:
AVAproj = AVArest + VC x (250-Qrest)
where AVArest is the resting AVA, Qrest is the resting mean transvalvular flow rate and VC is the ‘valve compliance’ or slope of the valve area – transvalvular flow relationship.82
In an initial 23 patients undergoing surgical inspection, AVAproj≤1.0 cm2 and an AVAproj≤0.55 cm2/m2 correctly classified 83 % and 91 % of patients, respectively.82 In a larger study of 52 patients, a simplified approach was used to calculate VC that only required the measurement of resting and peak DSE transvalvular flow rate and AVA:
VC = (AVApeak - AVArest)/(Qpeak - Qrest)
AVAproj≤1.0 cm2 correctly classified 92 % of patients, and was superior to DSE peak AVA<1.2 cm2 (77 %), peak MG>30 mmHg (73 %) and AVA increase <0.3 cm2 (60 %).83 However, AVAproj needs to be interpreted cautiously if the increase in transvalvular flow rate is <15 % due to inaccuracies in calculating VC.82
Quantitation of AVC by MDCT may provide an additional technique to potentially distinguish TSAS from PSAS, and be especially helpful in those patients in whom the DSE is non-diagnostic or the augmentation of transvalvular flow is minimal. In 18 patients with classical low-flow, low-gradient AS, AVC≥1,651 AU correctly classified 89 % of patients as having either TSAS or PSAS.84 Gender specific thresholds for identifying TSAS are likely warranted based on studies in AS patients with preserved LVEF (~1,250 AU in woman and ~2,050 AU in men), although this has not been studied in classical low-flow, low-gradient AS.41
Prediction of Clinical Outcome
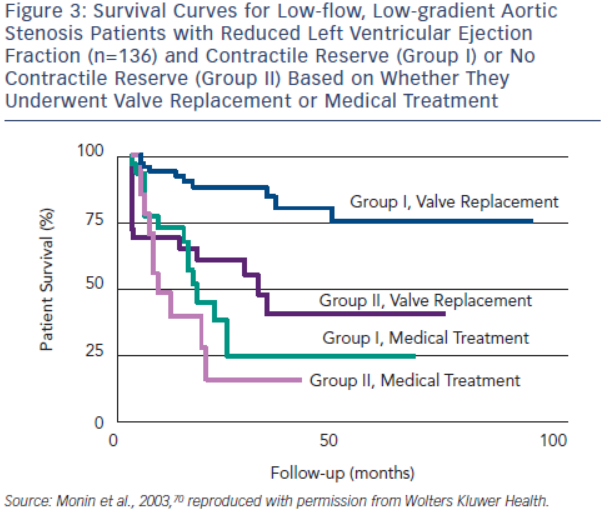
Contractile or flow reserve, defined as a 20 % increase in SV with DSE, is a strong predictor of peri-operative mortality rate and long-term survival.69,70 AVR is associated with a peri-operative mortality rate as high as 33 % in patients with no contractile reserve, but only 5–8 % in patients with contractile reserve.54,69,70 In the European Multicentre study, three-year survival of patients with contractile reserve undergoing AVR was 79 %, significantly better than the 35 % survival observed in patients with no contractile reserve (see Figure 3).70 Patients with contractile reserve did significantly better with AVR than medical management (three-year survival <25 %). Patients with no contractile reserve have a high operative mortality rate and poor long-term survival; however, their survival is better with AVR than medical therapy when tested using propensity matched analysis.54 Furthermore, patients with no contractile reserve have similar improvements in LVEF and New York Heart Association (NYHA) functional class following AVR as patients with contractile reserve.85 In essence, absence of contractile reserve identifies patients with a high operative mortality rate and reduced long-term survival who may still benefit from AVR. In the ESC guidelines, AVR should be considered in symptomatic patients with evidence of flow reserve (IIa recommendation) and may be considered in symptomatic patients without flow reserve (IIb recommendation).10
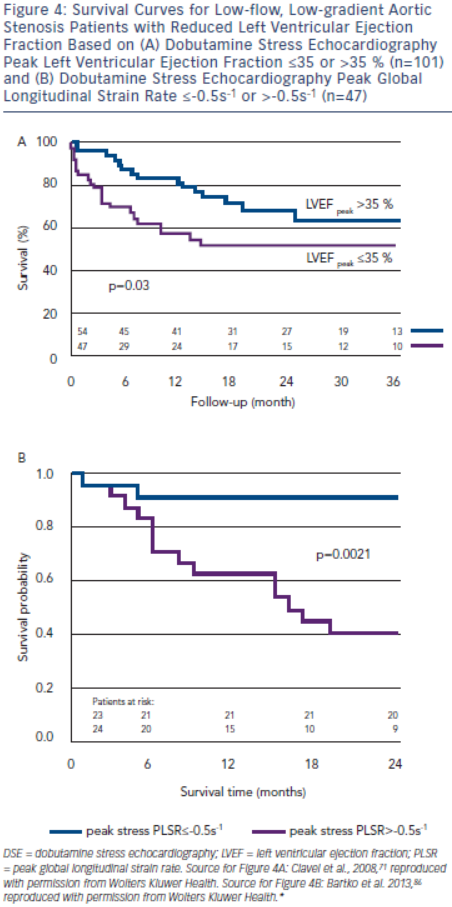
In the True or Pseudo Severe Aortic Stenosis (TOPAS) study of 101 patients with low-flow, low-gradient severe AS with reduced LVEF, independent echocardiographic predictors of reduced survival were AVAproj≤1.2 cm2 and DSE peak LVEF≤35 % (see Figure 4A).71,83 Contractile reserve was not an independent predictor of survival.71 The conflicting findings regarding the predictive value of contractile reserve in TOPAS and the European Multicentre Study may have related to different DSE protocols or differences in the study population (TOPAS had a greater prevalence of concomitant diseases such as coronary artery disease).70,71 Additionally, DSE peak LVEF is essentially a composite measure of both resting LVEF and ‘contractile reserve’, and may explain the better predictive value of this parameter.
Download original
Open in new tab
Longitudinal strain parameters are severely impaired in low-flow, low-gradient severe AS with reduced LVEF, but may improve with DSE in a substantial proportion of patients. In a substudy of TOPAS, patients with a DSE peak global longitudinal strain rate (PLSR) <-0.5 s-1 had a two-year survival of 91 %, but only 40 % if DSE PLSR was ≥-0.5 s–1 (see Figure 4B).86 DSE PLSR was the strongest predictor of overall survival and added incremental prognostic information to DSE peak LVEF.
Low-flow, Low-gradient Severe Aortic Stenosis with Preserved Left Ventricular Ejection Fraction
Definition
The criteria for low-flow, low-gradient severe AS with preserved LVEF include a VAS<4 m/s or MG<40 mmHg, AVA≤1.0 cm2, LVEF>50 % and SVi<35 ml/m2.9–11,19
True Versus Pseudo-severe Aortic Stenosis
Patients with low-flow, low-gradient severe AS with preserved LVEF have similar transvalvular flow rates as classical low-flow, low-gradient severe AS.83,87 Since transvalvular flow is reduced, the small AVA may reflect underlying TSAS or PSAS. The prevalence of PSAS is reported to be ≈30 % in these patients, similar to what is observed in classical low-flow, low-gradient severe AS.87 DSE and exercise echocardiography have been used to distinguish TSAS and PSAS in one small study.87 AVAproj≤1.0 cm2 was 94 % accurate in distinguishing the two conditions when compared to surgical inspection, and performed better than DSE peak MG≥30 mmHg (75 %) or ≥40 mmHg (81 %) and DSE peak AVA≤1.0 cm2 (69 %) or ≤1.2 cm2 (63 %). In the AHA/ACC and ESC valve guidelines, AVR is reasonable in symptomatic patients if the clinical, haemodynamic and anatomic data support valve obstruction as the most likely cause of the symptoms (IIa recommendation).9,10 Reserving surgical intervention only for symptomatic TSAS patients appears theoretically sound, although this management strategy has not been tested.
Measurements of AVC using MDCT may have a role in identifying TSAS and PSAS in patients with low-flow, low-gradient severe AS with preserved LVEF. In 451 patients with at least moderate AS, preserved LVEF, normal SVi and concordant MG and AVA measurements, an AVC threshold of 1,274 AU in women and 2,065 AU in men correctly classified 87 % of patients as having moderate AS or severe AS.41 A similar 87 % accuracy was obtained using an AVC indexed to aortic annulus area threshold of 292 AU/cm2 for women and 476 AU/cm2 for men. In a further 172 patients with preserved LVEF and discordant AS measurements (MG<40 mmHg and AVA≤0.6 cm2/m2), ~50 % of patients had an AVC score consistent with moderate rather than severe AS.41 Low flow (SVi <35 ml/m2) was present in ~16 % of these patients; however, results for this subgroup were not provided and DSE results were not reported. Further studies are warranted to determine whether AVC thresholds can be used to distinguish TSAS and PSAS in low-flow, low-gradient severe AS with preserved LVEF.
Prediction of Clinical Outcome
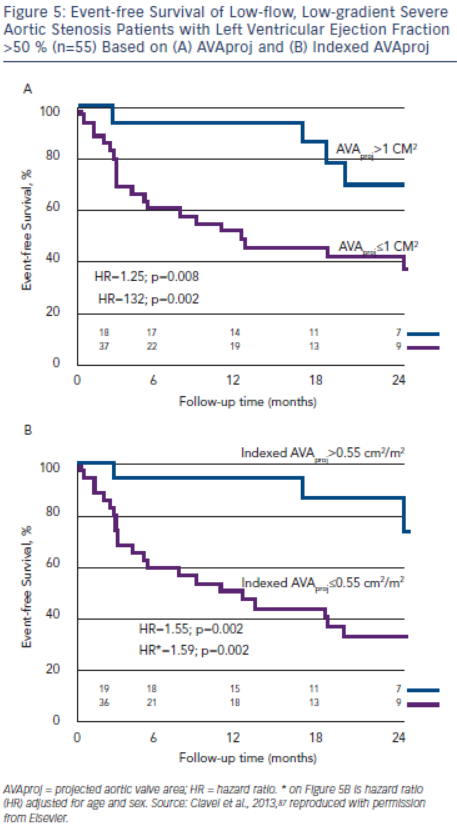
AVAproj and indexed AVAproj are strong predictors of adverse outcome in patients with low-flow, low-gradient severe AS with preserved LVEF (hazard ratio: 1.32 and 1.59, respectively, for each 0.1 cm2 decrease in AVAproj and indexed AVAproj) and is superior to traditional parameters of stenosis severity for predicting outcome.87 More than 90 % of patients with AVAproj>1.0 cm2 or indexed AVAproj>0.55 cm2/m2 remain free of death or AVR at 12 months, whereas only 50 % of patients with AVAproj≤1.0 cm2 or AVAproj≤0.55 cm2/m2 will be free of death or AVR (see Figure 5).87
Conclusion
The role of echocardiography in the evaluation of AS patients has progressed beyond the measurement of resting MG and AVA. Challenging situations in which patients have discordant haemodynamic data, asymptomatic haemodynamically severe AS or severe low-flow, low-gradient AS have resulted in new approaches to improve the haemodynamic evaluation, risk stratification and prediction of clinical outcome for patients with both normal-flow and low-flow AS. Integration of these advances will help ensure that AS patients receive the most appropriate management.







