Atherogenic dyslipidaemia encompasses a broad variety of lipid phenotypes. While LDL cholesterol is a well-known risk factor for atherosclerotic cardiovascular disease (ASCVD), there are additional atherogenic lipoproteins that may be targeted to further reduce ASCVD risk.
In their comprehensive review, Lorenzatti and Toth emphasise that, even when LDL cholesterol levels are optimised, ASCVD risk remains in a substantial subset of individuals.1 Some of this residual cardiovascular risk is due to suboptimal levels of other atherogenic lipids and lipoproteins, including triglycerides, HDL cholesterol, non-HDL cholesterol (total cholesterol minus HDL cholesterol), and apolipoprotein B (ApoB).
The 2018 American College of Cardiology/American Heart Association (ACC/AHA) Multi-Society Cholesterol Guideline and the recent 2019 European Society of Cardiology (ESC) guideline for the management of dyslipidaemias prioritise LDL cholesterol as the primary target of lipid-lowering therapy, principally with the use of maximally tolerated statin therapy.2,3 Both guidelines emphasise intense (≥50%) LDL cholesterol lowering and define specific values of LDL cholesterol to trigger additional recommendations. Moreover, if adequate LDL cholesterol reduction is not achieved despite lifestyle modifications and maximally tolerated statin therapy, consideration of non-statin therapy is warranted.
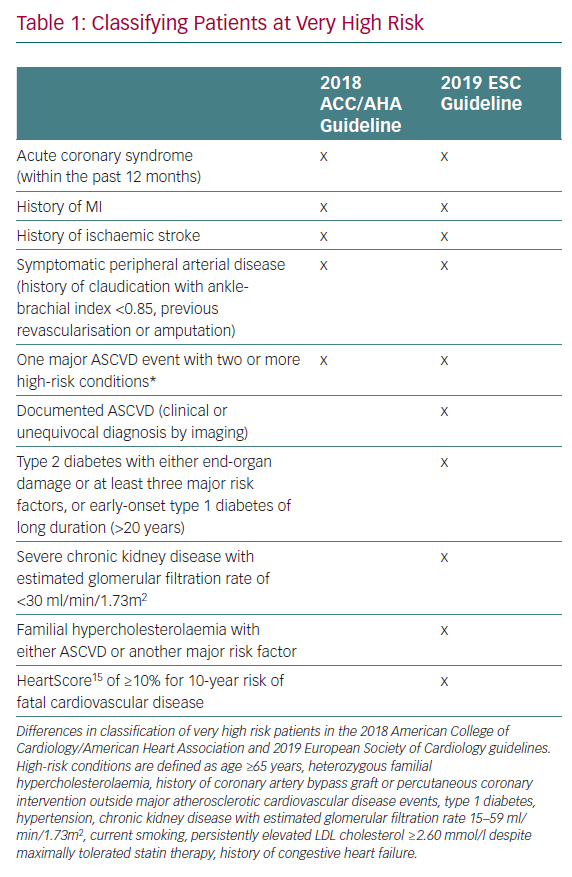
There are several key differences between the European and American guidelines, the first of which lies in the definition and treatment thresholds for very high risk patients. Table 1 outlines the similarities and differences in the definition of very high risk patients between the ACC/AHA and ESC guidelines. Second, in a departure from the ACC/AHA guideline, which recommends an LDL cholesterol threshold of 1.8 mmol/l before considering non-statin therapies, the ESC guideline recommends treating to a more aggressive therapeutic threshold of 1.42 mmol/l, thereby suggesting that non-statin therapies should be considered even where LDL cholesterol levels are 1.42–1.81 mmol/l.3 Finally, regarding several high-risk medical conditions, known to be risk-enhancing factors or risk modifiers, there are notable differences between the two guideline documents. As well as sharing many of the risk-enhancing factors described in the ACC/AHA guideline, the ESC guideline includes social deprivation, (central) obesity, physical inactivity, psychosocial stress, psychiatric disorders, HIV treatments, AF, left ventricular hypertrophy, chronic kidney disease, obstructive sleep apnoea and non-alcoholic fatty liver disease as risk modifiers.3,4
Both guidelines consider atherogenic lipoproteins beyond LDL cholesterol. Persistently elevated triglycerides (≥4.53 mmol/l) and elevated ApoB concentrations (≥3.37 mmol/l) are considered risk-enhancing factors in the 2018 ACC/AHA guideline. Their presence in intermediate risk or select borderline risk patients should inform the clinician-patient decision and facilitate shared decision making with regards to initiating or intensifying statin therapy.2 The ESC guideline recommends secondary goals for both non-HDL cholesterol (<2.20, 2.60, and 3.37 mmol/l) and ApoB (<1.68, 2.07, and 2.60 mmol/l) in individuals at very high, high, and moderate risk respectively. While no specific thresholds have been set for triglycerides, a triglyceride concentration <3.89 mmol/l is considered reasonable.3
Beyond the atherogenic lipoproteins already mentioned, there is another atherogenic biomarker that merits discussion. The association between elevated plasma concentrations of lipoprotein(a) [Lp(a)] and ASCVD is well established and there may be an emerging role for the assessment and treatment of elevated Lp(a) in clinical practice.5–10 Lp(a) levels ≥1.3 mmol/l or ≥125 nmol/l are considered a risk-enhancing factor in the 2018 ACC/AHA guideline and the presence of elevated levels can be used to reclassify ASCVD risk.2
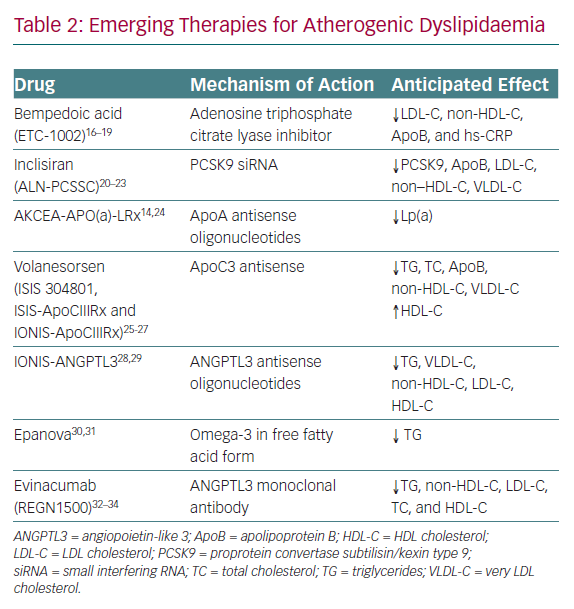
Currently, there are no evidence-based therapies to target elevated Lp(a) lowering, although some experts have advocated the potential use of niacin and/or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, which can modestly reduce plasma concentrations of Lp(a).11–13 An antisense oligonucleotide-based therapy directed at apolipoprotein(a) is in the late stages of development and is poised to be tested within the context of a randomised cardiovascular outcomes trial. A recent Phase IIB study demonstrated reductions of up to 80% in Lp(a) with this therapy.14 Beyond targeting Lp(a), a number of additional novel therapeutics for the treatment of atherogenic dyslipidaemia are on the horizon (Table 2).
While LDL cholesterol lowering has, understandably, remained the mainstay in the primary and secondary prevention of ASCVD, a comprehensive assessment of all atherogenic lipoproteins is merited. Mitigation of ASCVD risk should be targeted in the following manner: lifestyle modifications; targeting and surpassing LDL cholesterol therapeutic thresholds; and selective evaluation and treatment of additional measures of the atherogenic lipoprotein burden, including triglycerides, non-HDL cholesterol, ApoB and Lp(a).
The key to managing atherogenic dyslipidaemia lies in emphasising the foundational importance of therapeutic lifestyle changes and the apt usage of pharmacological agents. Fortunately, it appears that the effective therapeutic armamentarium is likely to increase. Meanwhile, we eagerly await the results of cardiovascular outcome studies testing several novel lipid-lowering therapeutics that have the potential to revolutionise the pharmacological management of atherogenic dyslipidaemia.