The treatment of patients with clinically significant chronic total occlusions (CTOs) will become more frequent with an ageing population, an increasing number of patients undergoing transcatheter aortic valve implantation (TAVI) (20 % have co-existing coronary disease) and patients presenting following coronary artery bypass graft (CABG) failure where redo CABG is not attractive or not possible.
The evolution of percutaneous coronary intervention (PCI) understanding and techniques has meant that these lesions can be treated percutaneously with a 90 % success rate in expert hands. Therefore, having access to a CTO programme with dedicated operators seems sensible.
Background
In clinical practice, coronary CTOs are defined as angiographic evidence of a total occlusion with thrombolysis in myocardial infarction (TIMI) grade 0 flow and an estimated or proven occlusion duration of more than 3 months.1
Contemporary practice in specialist CTO centres should expect success rates of 90 % for CTO PCI, which approaches success rates for non-occluded vessels. Despite this, the overall attempt rate for CTO lesions remains low, representing approximately 10–15 % of all PCIs that are performed for stable disease and only 5 % as a proportion of all PCIs across different healthcare systems.2,3
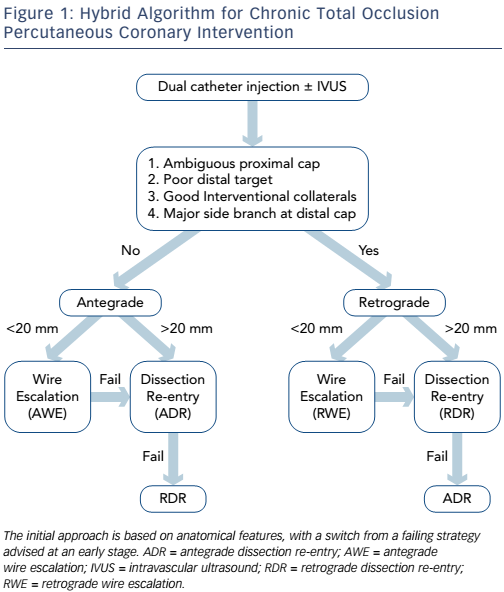
The hybrid approach to CTO PCI is a systematic algorithm-led PCI strategy based on the identification of key anatomical features on coronary angiography (see Figure 1).4 The main aim of this approach is to provide a standardised tool for training and programme development, avoiding futile strategies to improve safety, procedural success and reduce the contrast and radiation required to complete the case.
The hybrid algorithm utilises four techniques of crossing a CTO lesion:
- Antegrade wire escalation (AWE).
- Retrograde wire escalation (RWE).
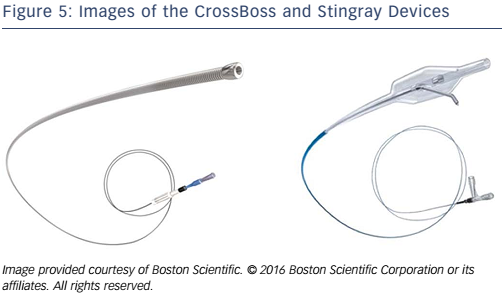
- Antegrade dissection re-entry (ADR) often using dedicated re-entry equipment (CrossBoss/Stingray; Boston Scientific).
- Retrograde dissection re-entry (RDR).
Each of these techniques has a success rate of around 80 % in isolation;5–7 however, when the techniques are used in combination higher overall success rates are obtained.8 Utilising only one or two of the strategies will limit overall success.
The anatomical features that dictate which technique is recommended include: proximal cap appearance, lesion length, distal landing zone and collateral channels (CCs).
Proximal Cap
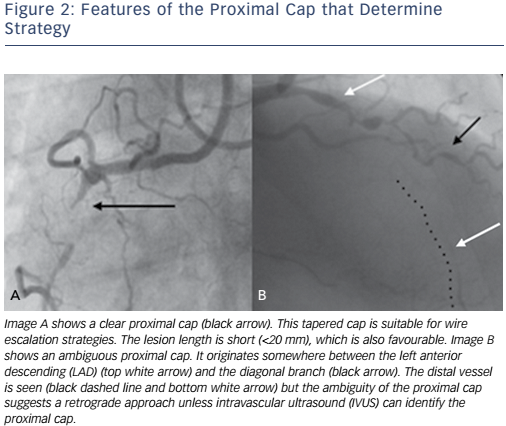
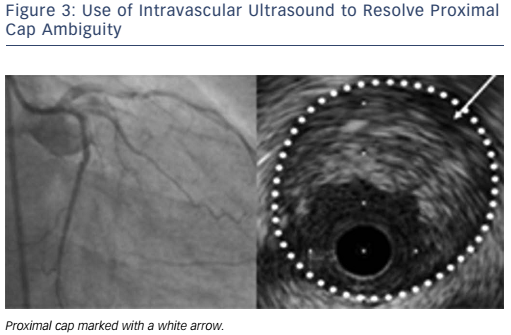
Is the proximal cap clear or ambiguous? An unambiguous proximal cap will favour an initial antegrade strategy whereas an ambiguous cap will often require retrograde techniques (see Figure 2). Intravascular ultrasound (IVUS) can be used to overcome ambiguity of the proximal cap (see Figure 3).
Lesion Length
Lesions that are <20 mm are generally of lower complexity and are commonly suitable for AWE as the primary strategy with high success rates.9
Distal Landing Zone
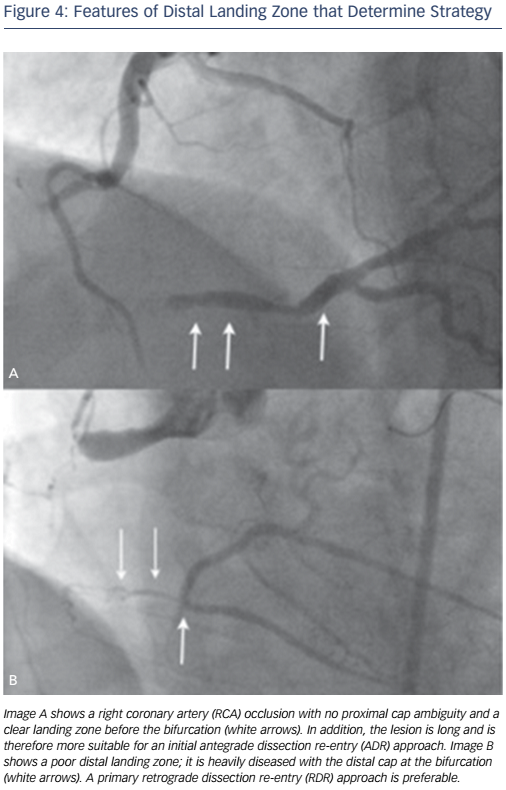
If the distal vessel is well-defined and remote from major side branches it may favour an ADR strategy. Whereas a distal cap at a heavily diseased landing zone or close to a bifurcation will not and RDR may be the initial strategy especially in the setting of interventional collaterals (see Figure 4).
Collateral Channels
Interventional CCs are ones that can be safely navigated with a wire and a microcatheter to access the distal vessel. This definition will vary according to operator experience and ability.
Assessing Complexity
The Japanese CTO score (J-CTO) is a simple and effective method of assessing anatomical complexity. A point is given for each of the five following variables:
- a blunt (versus tapered) proximal cap;
- lesion length >20 mm;
- calcification anywhere within the CTO segment;
- tortuosity defined as a >45° bend within the CTO segment; and
- if a prior attempt had been made.
When the J-CTO score was low, the CTO would be crossed in under 30 minutes, 90 % of the time. Whereas AWE was only successful at 30 minutes in 10 % of cases when the J-CTO score was high.9 The J-CTO score is a marker of complexity with high J-CTO score cases requiring more time, radiation and contrast, but does not strongly predict procedural success when dissection techniques (ADR/RDR) are available to experienced operators.10
We have demonstrated that educating and proctoring physicians on the hybrid algorithm and techniques leads to improved success rates. Physicians were more likely to undertake more complex lesions with higher success rates in both the low and higher complexity groups after dedicated training.11 This was mainly driven by an increase in success for patients with JCTO scores ≥2 (70.7 versus 49.5 %; p=0.0003).
Technical Approaches
Diagnostic Coronary Angiogram
The diagnostic angiogram should be taken with dual catheters unless the collateral filling is purely ipsilateral. A wide camera field of view is required with no panning and a long acquisition run to obtain complete information on collateral filling patterns, occlusion length and vessel course. Often collateral filling of the CTO can be compromised as a case progresses, therefore obtaining all the information at the start is important. Ideally the angiogram should be analysed prior to the procedure to completely understand the anatomy.
Antegrade Wire Escalation
The initial strategy of AWE is suitable when the proximal cap is unambiguous, the lesion length is short and the CTO segment is without significant tortuosity (see Figure 2A).
The initial setup for simple anatomy should be the same as for more complex anatomy with dual catheter angiography with guides in both vessels, using a larger calibre catheter in the target vessel (we recommend 8F) to facilitate a change in strategy.
Use of a microcatheter is recommended in all cases to facilitate guidewire exchange or tip re-shaping without losing position within the CTO segment.
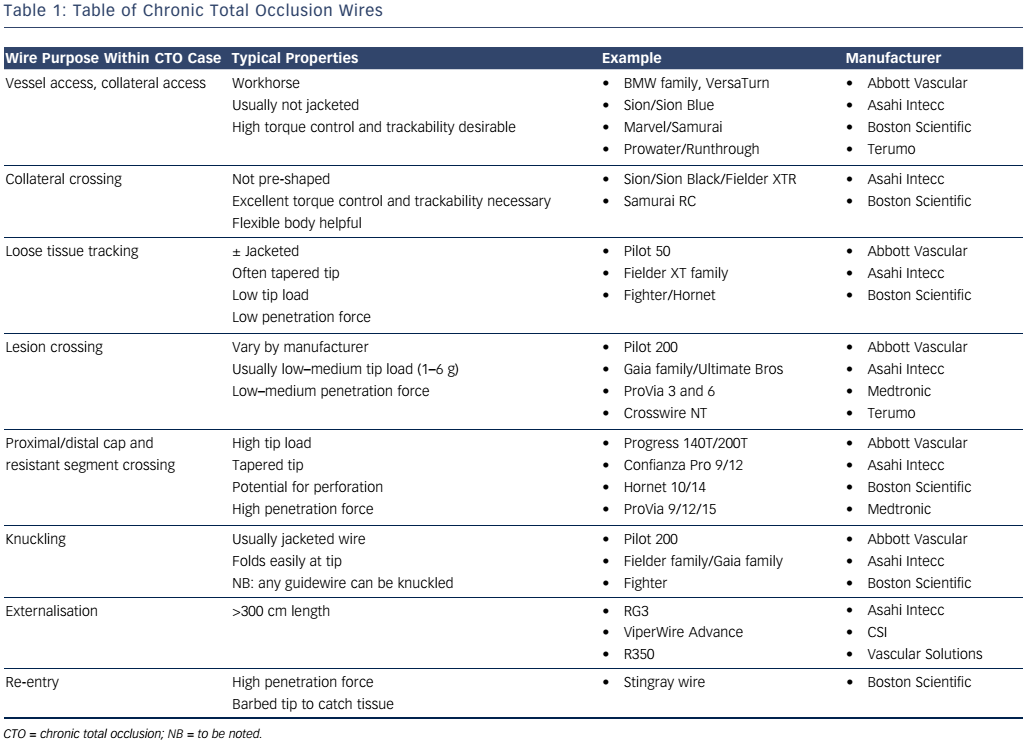
The initial wire for AWE should ideally be a tapered, polymer wire with a low tip load (see Table 1). These wires are designed for loose tissue tracking and are ideal for functional occlusions or short duration CTOs.12
If the initial wire does not cross, then escalating to a wire with more penetration force but good torque control and trackability is indicated (Gaia family; Asahi Intecc) (Pilot 200; Abbott Vascular).
The proximal cap is often calcified.12,13 If the initial wire fails to penetrate the proximal cap, operators should consider a wire designed to puncture through the proximal cap of high penetration force (see Table 1). However, as soon as the cap is punctured and the microcatheter passed beyond this barrier we recommend de-escalation to a second line wire (e.g. Gaia family or Pilot 200). Jacketed wires may be preferred if there is tortuosity within the CTO segment.
With wire escalation techniques, care should be taken to ensure that the wire is advancing in the correct path. When passing through the CTO body and especially approaching the distal cap, it is worth confirming in multiple angiographic views that the wire is within the correct vascular structure. The distal cap may be resistant just like the proximal cap, and a more penetrative wire may be required to puncture through. To confirm that the wire is within the true lumen distally, two orthogonal views should be taken with donor vessel (vessel supplying CCs) angiography and/or IVUS. A common safety step is to exchange out the specialist CTO wire for a workhorse wire using a microcatheter, to avoid the risk of distal vessel perforation.
In contemporary practice, most expert operators will use a microcatheter to cross a CTO. If the guidewire is noted to be in the incorrect position in the vessel, it can be removed and reshaped before re-use or else swapped for a different wire that has different properties and/or tip shapes. These ’redirection’ manoeuvres have the same aims as historical parallel wiring and have succeeded this technique for many operators. Ongoing manipulation of equipment within the CTO segment is likely to expand subintimal spaces and extend intramural haematoma. This will not only continually decrease the likelihood of wiring the distal lumen but will also vastly reduce the reliability of ADR as a bail-out. We have found contemporary ADR to be a much more reliable strategy for successful revascularisation and will default to this for failed AWE after redirection manoeuvres have failed.
Retrograde Wire Escalation
There are anatomical situations when approaching a CTO from a retrograde approach may be advantageous. When the CTO is ostial and/or the proximal cap is ambiguous, a retrograde approach will help overcome proximal cap ambiguity safely. When the distal cap involves a major bifurcation, a retrograde approach will more likely preserve both distal branches whereas antegrade strategies, especially ADR, may compromise a branch. Finally, if an antegrade CTO attempt is unsuccessful, a bail-out retrograde approach may still be successful.
There are also differences in pathological composition between the proximal and distal cap. More frequently, the distal cap is tapered rather than blunt.12 This favours wire-based strategies and RWE is often successful for shorter lesions when retrograde access is achieved.
Retrograde procedures are technically more challenging, with several additional features requiring specific attention, which include safe crossing of CCs, careful monitoring of anticoagulation, appropriate guide catheter selection (both in terms of diameter and the support offered) as well as familiarity with a range of microcatheters and their manipulation.
Once a microcatheter is positioned at the distal cap a similar wire escalation approach is used for RWE as described for AWE. Initially a Fielder XT-A (Asahi Intecc) can be considered but if this fails to progress or buckles then escalation to a stronger lesion crossing wire should be considered to penetrate the distal cap (see Table 1).
CCs will be discussed in a later section.
Antegrade Dissection and Re-entry
There are two common scenarios where this strategy is utilised. If AWE has been attempted but has failed due to subintimal wire passage beyond the distal cap and failure to re-enter the distal lumen, and where lesions are long and complex meaning that AWE has a high possibility of failure and that ADR will potentially lead to a safer and more efficacious procedure for the patient, particularly in the absence of retrograde options.
This second scenario led to the evolution ADR strategies. As many as 50 % of all cases may have no retrograde option due to the type, size, course or lack of an interventional CC.14 Even when a case is selected for an initial retrograde approach, experienced operators fail to get a wire across the CCs 20 % of the time.5
Early antegrade dissection techniques (subintimal tracking and re-entry (STAR), mini-STAR) were associated with high rates of target vessel revascularisation and re-occlusion due to unselected re-entry often at point of bifurcation with compromise of some distal branches.15–17 Nevertheless, the concepts of these early techniques have led us to contemporary ADR.
The CrossBoss and Stingray system (Boston Scientific; see Figure 5) have been developed as a combination of devices that can create a controlled antegrade dissection plane to facilitate targeted distal vessel re-entry. The CrossBoss catheter is a blunt dissection tool with a 1 mm rounded tip. The CrossBoss will either track through intimal plaque and re-enter the distal true lumen itself or create a controlled dissection plane in the subintima that allows the Stingray balloon to be delivered just beyond the distal cap of the occlusion. The Stingray balloon is then inflated to wrap itself around the artery lumen. This allows a purpose-built wire to re-enter in a predictable and controlled position of the distal artery. When the vessel is reconstructed with stents, outflow is therefore guaranteed to multiple branches.18
The ideal lesion for ADR as a primary strategy would be one with a defined proximal cap, where there is a long CTO segment with a good target vessel that is clear of major bifurcations (see Figure 4A). There are several principles that should be applied to these procedures. The proximal cap anatomy must be safely defined (angiographically or with a supplementary IVUS). The CrossBoss can struggle with proximal cap penetration and it may be necessary to penetrate the proximal cap and then modify the proximal cap with a microcatheter or focused balloon inflation. Once the proximal cap has been crossed with any equipment, antegrade contrast injections must be avoided to prevent hydraulic dissection distally into the vessel and subsequent loss of the landing zone.
The CrossBoss should be advanced ahead of a guidewire into the distal vessel using a fast spin technique until it passes just beyond the distal cap. At this point, re-entry should be attempted. The CrossBoss should be removed, with a guidewire left in position and the Stingray balloon introduced into the vessel and passed to the same position. It is imperative that re-entry occurs at a site proximal to any bifurcation to preserve all distal branches.
The Stingray balloon must be meticulously prepared to ensure that it is visible radiographically and that it can be orientated correctly to facilitate safe and controlled re-entry. Once introduced into the correct position in the vessel, the Stingray should be inflated to4–6 atmospheres and then orientated to understand if the target vessel is above or below the balloon by using contralateral contrast injection. At this point, re-entry is performed by advancing the dedicated Stingray wire back into the true lumen of the vessel. This is a penetrative wire with a barb at its tip designed to catch and penetrate tissue (see Figure 5). The distal vessel can be wired directly with the Stingray wire or more commonly changed for a more controllable wire (e.g. Pilot 200).
Once the distal vessel is secured, the Stingray balloon should be deflated and removed. As a safety step, we would suggest using a microcatheter to exchange the stingray wire for a workhorse guidewire. The vessel can then be predilated and stented. Care should be taken with stent sizing, recognising that stents may be deployed in the subintimal space. A balance must be struck between obtaining a luminal area that will provide a good long-term outcome versus oversizing stents and risking a perforation.
Retrograde Dissection and Re-entry
A RDR approach is recommended when there is an ostial occlusion, proximal cap ambiguity that cannot be resolved safely, when the distal cap involves a bifurcation (to preserve all branches) or when an antegrade approach has been unsuccessful (see Figure 4B) and when there are ’interventional collaterals’ that in the experience of the operator can be used to obtain retrograde access to the distal target vessel. The rationale for adopting a RDR approach is procedural safety and efficacy.
With long, tortuous occlusions the risk of wire-based perforations with stiff tapered guidewires is higher. When blunt force is applied to the artery using a knuckled (or folded) polymer jacketed wire the risk of exiting from the vessel architecture is low. This theory is analogous to cardiac device implanters using their hands to bluntly dissect and therefore safely separate tissues when forming a pacemaker pocket, rather than using a scalpel or scissors.
The main limitation of retrograde approaches is often the inability to cross an interventional CC. Additional modes of failure (equipment not crossing, failure to complete) means that the overall failure with an initial retrograde approach is approximately 25 % of pre-selected cases.5
The original use of a retrograde approach with dissection was described in Japan as the controlled antegrade and retrograde tracking (CART) technique, when a balloon was brought retrogradely to facilitate antegrade wire passage. CART has now been largely superseded by the reverse CART (rCART) technique. The advent of modern microcatheters has meant that these are passed into the target vessel retrogradely in preference. Antegrade equipment is introduced into the vessel and wires are passed into the CTO segment from both directions. A balloon is introduced on the antegrade wire and inflated within this common subintimal space, thus allowing the retrograde wire to be directed via this segment into the proximal true lumen.
Frequently, guide catheter extensions are used in the antegrade system and brought as close to the retrograde equipment in the dissection as possible. This minimises the distance the retrograde wire has to travel to complete the case and increases efficiency and reduces complications.19
RDR procedures are complex and involve multiple steps. They are associated with increased complication rates compared with AWE procedures. We would suggest that operators undertake dedicated education and training before embarking on this type of intervention. Nevertheless, these techniques can be learnt and effectively taught with good outcomes produced from training programmes.11
Collateral Channels
There are three types of retrograde channels to access the distal vessel: bypass graft, septal and epicardial collaterals. It is important to consider the CC size, take-off, course and exit.
The initial Rentrop system20 has been modified21 and is used to describe CCs: CC grade 0; no continuous connection, CC1; thread-like continuous connection and CC2; side branch-like connection. While the classification is descriptive it does not correlate well with crossing success and sometimes CC0 collaterals are crossable especially with modern wires.
Septal CCs are preferred mainly for patient safety and should be used preferentially, especially early in the learning curve. The septum is more forgiving in terms of equipment-induced vessel injury; this usually results in localised haematoma rather than perforation and tamponade. It is also possible to dilate septal CCs whereas it is not advisable to dilate the epicardial vessels due to risk of perforation. Septals are, however, often tortuous and complex connections and it is possible to spend prolonged periods of time failing to cross a septal CC. Epicardial connections are usually more predictable in terms of understanding whether crossing is feasible. However, small calibre, friable epicardial CCs should be avoided until the operator is very experienced.
To enter a CC, a workhorse wire with a significant primary bend is initially used through the microcatheter. Once the CC has been engaged the microcatheter is introduced and the wire is exchanged for a specialist collateral crossing wire (see Table 1) with a short (1 mm) and subtle primary bend (<40°). Septal CCs can be crossed by ’surfing’. This is a blind, repetitive wiring technique, where the wire is passed forwards until resistance is felt. It is then withdrawn slightly and re-directed until it passes into the distal vessel. The other technique is to perform ’tip injections’ with neat contrast through the microcatheter to understand the CC course in detail, ensuring there is no air in the catheter and that blood can be withdrawn. Tip injections are often needed in epicardial CC crossing as the microcatheter frequently obstructs contrast passage. It is important to ensure microcatheters are not advanced over the wire until the operator is certain that they are safely within the CC or distal target vessel.
Occasionally the microcatheter fails to advance due to tortuosity or size of the CC. If it is a septal CC the microcatheter should be removed using a trapping technique. Trapping will prevent the need for prolonged fluoroscopy for equipment exchanges. Where trapping is not possible, wire extensions or 300 cm wires are required. A small diameter balloon can be used to dilate the septal track (usually 1.2–1.5 mm with longer balloon lengths preferred) and nominal pressure balloon inflation is sufficient. This must not be performed in epicardial CCs. If septal dilation fails or epicardial CCs are not crossed with a Corsair or Turnpike, a lower profile microcatheter (Turnpike LP, Vascular Solutions Inc; Caravel, Asahi Intecc; Finecross, Terumo, etc.) may facilitate passage.
Conclusion
Based on historical data the number of CTO procedures performed are much lower than would be reasonably expected. The treatment of patients with clinically significant CTOs will become more frequent with an ageing population, an increasing number of patients undergoing TAVI (20 % have co-existing coronary disease) and patients presenting following CABG failure where redo CABG is not attractive or not possible. Meanwhile understanding and techniques have developed such that 90 % success can be expected in expert hands. Therefore, having access to a CTO programme with dedicated operators seems sensible.