In December 2019, several cases of interstitial pneumonia of unknown origin were detected in Wuhan, China, and on 9 January 2020, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified as the causative agent of coronavirus disease 2019 (COVID-19). Within a few weeks, the contagion spread through China and South Korea, and the outbreak rapidly extended worldwide due to asymptomatic cases and modern travel. Finally, on 11 March 2020, the WHO declared the coronavirus outbreak a pandemic.
Over the past few months, health systems worldwide have been put to the test with the uncontrolled diffusion of a completely new disease with different levels of severity, and frequently, poor prognosis. Even though the leading clinical manifestations of SARS-CoV-2 infection involve the respiratory tract, with a risk of worsening dyspnoea, oxygen blood desaturation and frequent need for orotracheal intubation, the involvement of other organs and systems, such as the cardiovascular system and the gastrointestinal tract, can lead to multiorgan failure with fatal consequences.
The mechanisms underlying the pathophysiology of COVID-19 and its systemic manifestations are not well understood. The SARS-CoV-2 host cell receptor, the angiotensin-converting enzyme 2 (ACE-2), is widely expressed in several organs, including the lung, heart, endothelial cells, kidney and intestine, thus explaining the detection of viral particles in cardiac pericytes and urinary and faecal samples.1 Of importance, host immune system dysregulation, which is a consequence of viral infection, has recently been found to be important in determining exaggerated cytokine release and inflammasome activation, which are respiratory and non-respiratory consequences of the disease.2 The proinflammatory milieu may contribute to microvascular dysfunction and diffuse intravascular coagulation.3
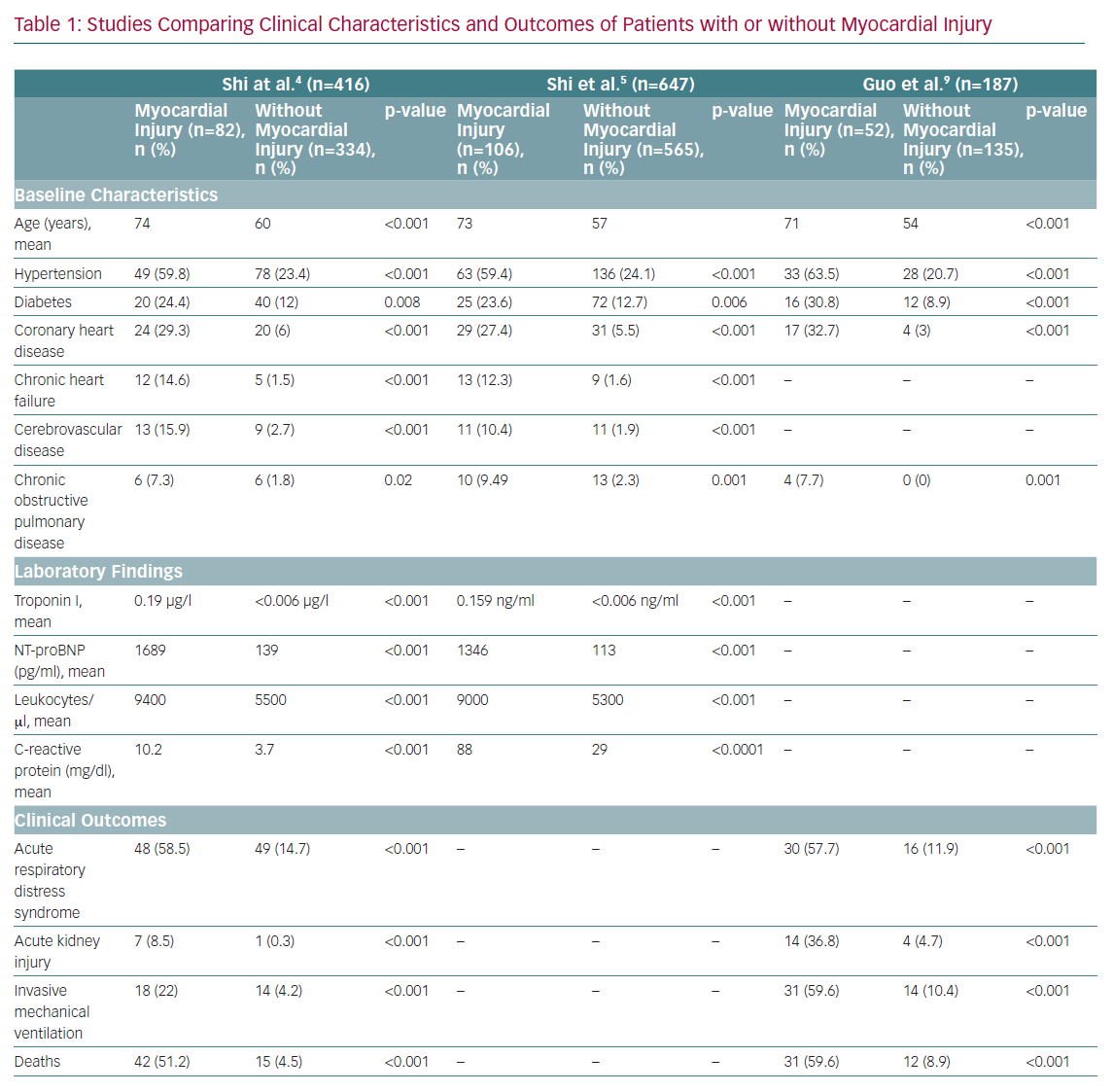
The cardiovascular system is often involved in SARS-CoV-2 infection, and patients with underlying cardiovascular disease (CVD) or with new onset cardiovascular complications need prompt diagnosis and treatment, as their prognosis is usually poorer (Table 1).
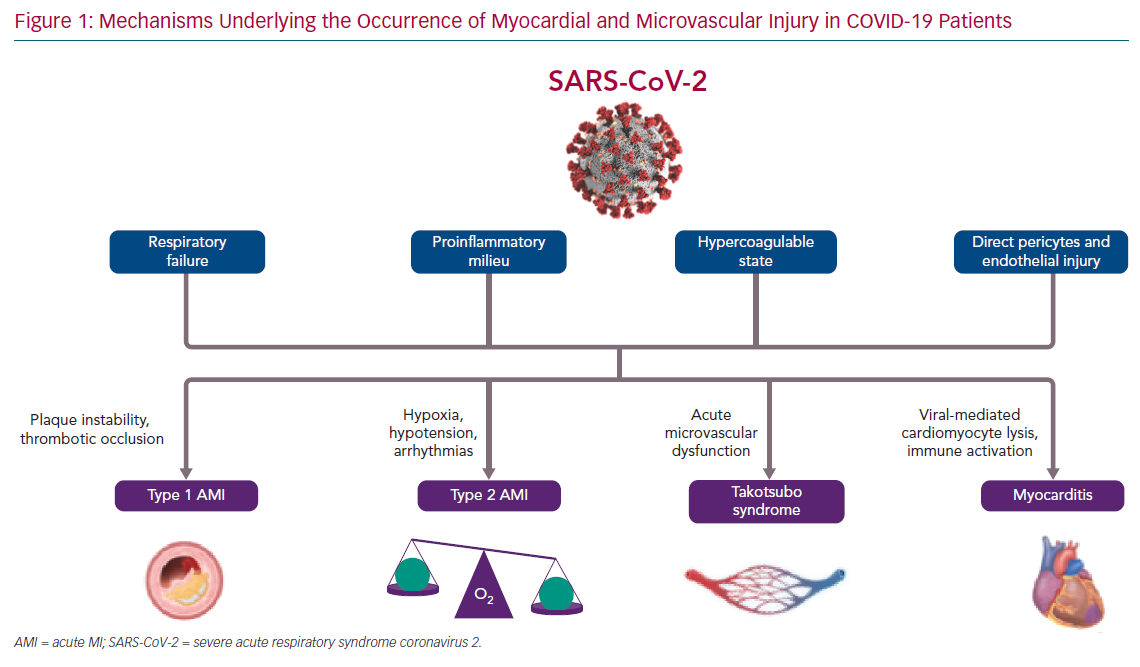
Herein, we discuss the direct and indirect mechanisms leading to myocardial and microvascular injury in SARS-CoV-2 infection (Figure 1) and the implications of the COVID-19 pandemic for patients and clinicians in terms of CVD management and treatment.
Pathogenic Mechanisms Underlying Myocardial and Microvascular Injury
Patients with COVID-19 can develop cardiovascular complications, such as heart failure, myocarditis, pericarditis, vasculitis and cardiac arrhythmias. Moreover, between 8% and 28% of patients with COVID-19 will manifest troponin release early in the course of the disease, reflecting the occurrence of a myocardial cardiac injury.4 Of note, the presence of troponin elevation, or its dynamic increase during hospitalisation, confers up to a fivefold risk of requiring ventilation; increases in arrhythmias, such as VF and ventricular tachycardia; and a fivefold risk of mortality. In their study, Shi et al. showed that, among 671 SARS-CoV-2-positive patients, those who died (n=62) were more likely to have myocardial injury (75.8% versus 9.7%; p<0.001) than those who survived, thus suggesting that markers of myocardial necrosis may be useful in predicting the risk of in-hospital death.5 Of importance, the area under the receiver operating characteristic curve of initial cardiac troponin I for predicting in-hospital mortality was 0.92 (95% CI [0.87–0.96], sensitivity: 0.86, specificity: 0.86, p<0.001), and in the multivariable logistic regression, older age, comorbidities (e.g. hypertension, coronary heart disease, chronic renal failure and chronic obstructive pulmonary disease), and a high level of C-reactive protein were predictors of myocardial injury.
Myocardial injury could be due to different pathogenic mechanisms (ischaemic or non-ischaemic), and thus have different clinical consequences.4 In particular, cardiac ischaemia can arise from an imbalance between oxygen supply and demand, a type 2 MI, a situation that can prevail in acute infections, particularly those that affect the lungs, such as COVID-19. As with other pneumonias, cardiac troponins are often increased in COVID-19 as a result of pre-existing CVD and/or the acute stress related to the viral infection, thereby mild elevations in patients without signs or symptoms of heart involvement nor ECG changes do not require further investigation. However, cardiac biomarker levels correlate with disease severity and prognosis, so attention should be paid to increments that are two to three times the upper limit, which could be associated with typical angina and/or new-onset ECG abnormalities as possible markers of cardiac ischaemic or non-ischaemic damage.6 Type 2 MI is typically related to imbalanced myocardial oxygen demand and supply, and several different mechanisms could lead to this complication in COVID-19 patients. For example, the hypotension determined by the septic state and the blood hypoxemia as a consequence of respiratory function impairment could reduce oxygen supply to the heart, thus leading to acute myocardial injury, particularly in patients with underlying chronic coronary syndrome.7 Moreover, increased myocardial oxygen demand could be a consequence of sustained/repetitive cardiac arrhythmias, which has been reported in 16.7% of all hospitalised COVID-19 patients and in 44.4% of patients requiring intensive care admission to date.8
However, some patients could present with a type 1 MI due to pre-existing coronary plaques (often present in older patients with hypertension and/or multiple comorbidities; the majority of patients admitted to hospital for COVID-19) that become unstable because of inflammatory activation due to SARS-CoV-2. The proinflammatory milieu determined by viral activity can cause a destabilisation of coronary artery plaques.9 Moreover, the procoagulatory state favours coronary artery thrombotic occlusion.3
Microvascular dysfunction is another mechanism responsible for myocardial injury in SARS-CoV-2-positive patients. Microvascular impairment in these patients can be a consequence of the exaggerated systemic inflammatory response and the relied endothelial dysfunction found to be responsible for microthrombi formation. In addition, a direct action of SARS-CoV-2 on microvessels has been suggested, as the cellular host receptor ACE-2 is also expressed on the surface of endothelial cells.10 In their study, Chen et al. demonstrated that heart pericytes have a high expression of ACE-2, thus endorsing the hypothesis that pericyte injury due to virus infection could result in capillary endothelial cell dysfunction and microvascular impairment.1 It is also well known that acute coronary microvascular dysfunction can contribute to the onset of takotsubo syndrome (TTS).11,12 At present, a few cases of TTS have been registered among COVID-19 patients; therefore, the prevalence and the prognostic impact of this syndrome in this particular patient population is still unknown.13
At the opposite end of the spectrum, myocardial injury could be a consequence of myocardial involvement in inflammatory processes leading to myocarditis. Of note, in a case series of 150 patients with COVID-19, in which 68 deaths were reported, 7% were attributed to myocarditis with circulatory failure.14 The degeneration of cardiomyocytes and inflammatory infiltrates in the myocardial interstitium have been described together with vascular disepithelisation, vasculitis and microthrombi formation. However, SARS-CoV-2 has still not been isolated in the myocardium, thus experts are unable to determine whether myocarditis in COVID-19 patients is only an epiphenomenon of the infection-related systemic inflammatory response or if it could also be the direct consequence of local viral activity.15
Clinical Implications
Severe respiratory distress is usually considered as a leading cause of SARS-CoV-2-induced death. Other complications are also associated with increased risk of in-hospital death. Although information on cardiac complications is limited among COVID-19 patients, cardiac impairment has been demonstrated as a direct or underlying cause of death in 27% of pneumonia-associated deaths. Even after adjustment for baseline risk, cardiac complications are associated with a 60% increase in pneumonia-associated short-term mortality.16,17 The occurrence of myocardial injury in particular has been shown to be associated with higher mortality among COVID-19 patients.9
A highly fatal possible consequence of myocardial injury due to SARS-CoV-2 is the development of cardiogenic shock (CS), defined as clinical and biochemical evidence of peripheral hypoperfusion. In COVID-19 patients in the intensive care unit, CS of undetermined aetiology occurs in up to 12% of cases, and the management of this ominous complication is critically time dependent and requires multidisciplinary expertise.18 Possible causes leading to CS in these patients are ST-elevation MI (STEMI), myocarditis, stress cardiomyopathy, and acute heart failure as a consequence of respiratory and renal impairment; however, sepsis should also be considered as a possible or mixed aetiology. In this patient population, the potential beneficial and harmful effects of mechanical circulatory support (MCS) should be properly weighted to consider the procoagulatory state characteristic of COVID-19 and the need for specific treatments for lung injury, including the prone position. In critical cases, when MCS is undeferrable, venoarterial extracorporeal membrane oxygenation (VA-ECMO) is the strategy of choice, as it guarantees both lung and cardiac function support. Impella and intra-aortic balloon pump can be useful for left ventricular overdistention management in patients receiving VA-ECMO, and the latter should be considered as an alternative option in patients presenting with STEMI-related mechanical complications if other MCS are not available.18
Clinicians should be aware that there are multiple pathogenic mechanisms underlying myocardial injury and a differential diagnosis is not easy. In a recent study, Stefanini et al. demonstrated that in approximately 40% of SARS-CoV-2-positive patients undergoing urgent coronary angiography for suspected ST-STEMI a culprit lesion was not detected, and other mechanisms should be investigated (i.e. type 2 MI, TTS and myocarditis).19
In particular, if myocarditis is suspected, cardiac magnetic resonance might be considered for further diagnostic assessment, whereas endomyocardial biopsy is not recommended. However, there are no clear guidelines for SARS-CoV-2-related myocarditis treatment, but anti-inflammatory drugs have proved beneficial when associated with supportive therapies.6
The management of COVID-19 patients presenting with acute coronary syndrome has been matter of debate in recent months. The latest European Society of Cardiology (ESC) guidance for the management of cardiac complications related to COVID-19 suggests that, in case of STEMI, timely primary percutaneous intervention should be performed as stated by current ESC guidelines, irrespective of SARS-CoV-2 infection, and in the absence of virological testing results available at the time of the procedure, every patient should be considered as positive. Immediate complete revascularisation when non-culprit lesions susceptible of treatment are present should be considered if appropriate to avoid staged procedures and to reduce hospital stay.18 Fibrinolysis remains the strategy of choice when percutaneous intervention is not feasible within 12 hours of symptom onset. In case of non-ST-STEMI, the treatment strategy should be based on risk stratification. In very high-risk cases, immediate invasive strategy should be performed; in high-risk cases, SARS-CoV-2 testing should be delayed if possible; and in intermediate/low-risk cases, differential diagnoses and non-invasive strategies, such as coronary computed tomography angiography, should be prioritised and appropriate follow-up should be planned.18
Finally, in order to reduce the occurrence of myocardial injury among COVID-19 patients, the use of medications improving microcirculation has been recently suggested. This strategy could be particularly relevant for vulnerable patients with risk factors for pre-existing endothelial dysfunction (i.e. male sex, smoking, hypertension, diabetes, obesity and established CVD).20
In conclusion, the occurrence of myocardial injury in COVID-19 patients is a frequent and prognostically relevant complication. Clinicians should be aware that multiple mechanisms could explain this phenomenon, and clinical management may differ according to the underlying mechanism.