Stable angina pectoris is the most prevalent clinical manifestation of coronary heart disease. While the overall prognosis in patients with stable angina is good, with a low yearly event rate of ~1–2 %,1 for many, adequate symptom control can be difficult to achieve, leading to significantly impaired quality of life.
The traditional approach to the pharmacological management of stable angina, as advocated by European Society of Cardiology (ESC), American Heart Association/ American College of Cardiology (AHA/ACC), and National Institute for Health and Care Excellence (NICE) guidelines, follows a stepwise algorithm based on categories of first- and second-line anti-angina drugs for all patients.1–3 However, none of the first- or second-line drugs used to treat stable angina symptoms have been shown to reduce cardiovascular mortality or the rate of myocardial infarction (MI) when evaluated in clinical trials. Clinical trial data showing the superiority of any one anti-anginal drug over another is, similarly, lacking.
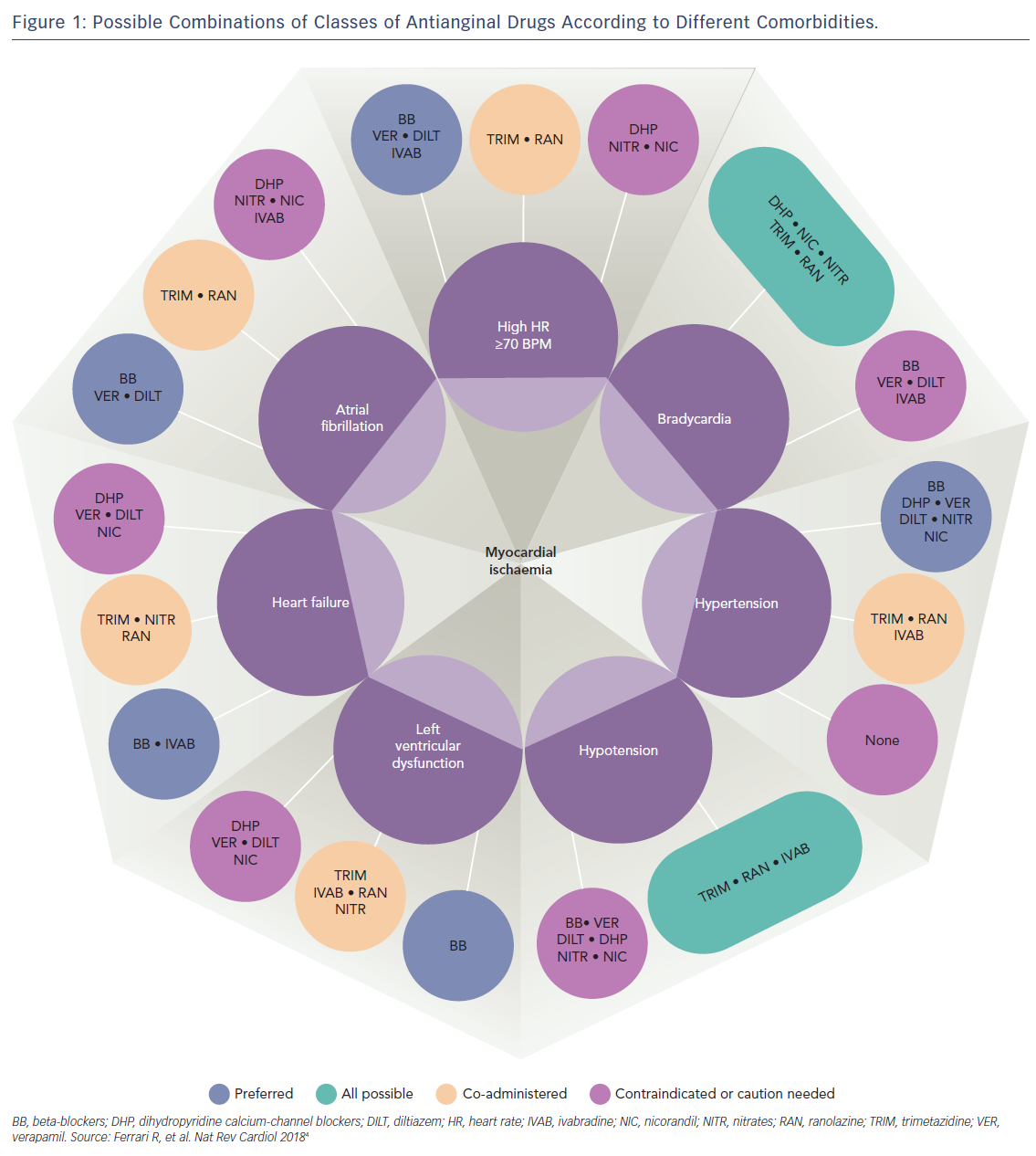
Therefore, because all available anti-angina drugs are equally effective, an alternative mechanistic-based approach to drug selection based on individual patient factors has been proposed (Figure 1).4 Importantly, this new approach recognises the multifactorial aetiology of stable angina, which includes not only typical effort-induced angina arising from obstructive epicardial artery disease, but also microvascular angina (where coronary blood flow abnormalities occur in the absence of epicardial artery stenoses), and vasospastic angina due to episodic vasoconstriction of both atherosclerotic and unobstructed coronary arteries.
In this proposed scheme based on expert consensus, vasodilatory drugs – including nicorandil and nitrates – are preferred over beta-blockers and other rate-limiting anti-angina drugs for patients with effort-induced angina and a low resting heart rate or atrioventricular conduction defects, and are avoided in patients with low systemic blood pressure who are most susceptible to the haemodynamic side-effects of these medications.
Although calcium-channel antagonists are the drugs of choice for the treatment of angina resulting from coronary artery spasm, vasospastic angina can also be successfully treated with both nicorandil and nitrates.5,6 These vasodilator drugs are also useful for some patients with mixed angina (who experience symptoms of both typical effort-induced angina and coronary vasospasm), as well as those with microvascular angina.7 However, long-acting nitrates are effective in only ~50 % of patients with microvascular angina8 as, unlike nicorandil, they have little effect on small resistance vessels.9
This article provides a focused update on the use of nicorandil and long-acting nitrates for the treatment of stable angina.
Nicorandil
Nicorandil is a balanced vasodilator, with dual mechanisms of action as both a nitric oxide (NO) donor and K+ATP channel agonist. Its chemical structure – N-[2-(Nitro-oxy) ethyl]-3-pyridine carboxamide – consists of a nicotinamide derivative combined with nitrate moiety. Nicorandil undergoes denitration and bioactivation via the nicotinamide/nicotinic acid pathway.10 The nitrate-like action of nicorandil possibly accounts for the majority of its clinical efficacy in angina, which is mediated via NO activation of cyclic guanosine-3’,-5’-monophosphate (cGMP) signaling pathways within vascular smooth muscle cells, causing peripheral venous and coronary arterial vasodilatation.11,12
On average, a single dose of nicorandil 20 mg results in a 10–15 % increase in mean luminal diameter of the epicardial coronary arteries.13 In addition, nicorandil causes significant vasodilatation of the coronary microvasculature and peripheral resistance arteries.14,15 These haemodynamic changes offload the ventricles through reductions in both preload and afterload, and improve coronary blood flow.16 In some patients, a mild baroreceptor reflex tachycardia occurs in response to vasodilatation. However, unlike several other anti-angina drugs, nicorandil does not affect cardiac conduction or contractility. Although nicorandil is used as a treatment for stable angina in many countries, it is not currently licensed in the US.
Dosage and Pharmacokinetics
The usual starting dose of nicorandil is 10 mg twice daily (5 mg for patients susceptible to headache). While this dosage can be uptitrated to 20 mg or a maximum of 30 mg twice daily, the lowest effective dose is recommended to avoid potential side-effects, particularly in the elderly. Nicorandil is rapidly absorbed via the gastrointestinal tract, with >75 % oral bioavailability as it does not undergo first-pass metabolism. Nicorandil exhibits a linear dose-to-plasma concentration, and reaches a maximal plasma concentration after 30–60 minutes, and steady-state levels after 4–5 days. Gastric absorption is delayed by food, but its pharmacokinetic properties are not significantly affected by age, chronic liver disease or chronic kidney disease. The desired clinical effects of nicorandil persist for ~12 hours, hence the need for twice-daily dosing.10,17 Nicorandil is eliminated mainly in the urine as metabolites N-(2-hydroxyethyl)-nicotinamide, nicotinuric acid, nicotinamide, N-methyl-nicotinamide and nicotinic acid, with a half-life of ~2 hours for the main phase of elimination.
Clinical Efficacy Data
Nicorandil was shown to significantly reduce the frequency of angina episodes and improve exercise capacity in several small placebo-controlled studies performed in the late 1980s.18-22 Subsequent short-term studies demonstrated that the nicorandil was similarly effective for angina prophylaxis as other conventional anti-anginal drugs, including beta-blockers,23-25 calcium-channel antagonists26,27 and long-acting nitrates.28,29 The Study of Nicorandil in Angina Pectoris in the Elderly (SNAPE) study found that there were similar improvements in both the time to angina and ST-segment depression during symptom-limited bicycle exercise testing after 4 weeks of treatment with nicorandil and isosorbide mononitrate compared to placebo.30 Similarly, the Comparison of the Antiischaemic and Antianginal Effects of Nicorandil and Amlodipine in Patients with Symptomatic Stable Angina Pectoris (SWAN) study showed comparable increases in time to angina and exercise capacity, and a reduction in the magnitude of ST-depression for nicorandil and amlodipine.31
Does Nicorandil Improve Prognosis?
Data from two trials suggest that nicorandil might confer modest improvements in clinical outcomes for patients with stable angina; however, this prognostic benefit has yet to be proven conclusively.
The Impact Of Nicorandil in Angina (IONA) study was a randomised placebo-controlled trial of 5,126 patients with stable angina followed up for an average of 1.6 years, which showed a reduction in the composite endpoint of death caused by coronary heart disease, non-fatal MI or unplanned hospital admission with chest pain in patients treated with nicorandil compared to placebo (HR 0.83, p=0.014).32 While there was no evidence of heterogeneity of benefit from nicorandil across subgroup status in the IONA study,33 there was also no difference in the secondary outcome of coronary heart disease death or non-fatal MI, and the individual components of the composite endpoint did not differ significantly between the two treatment groups.34 Nicorandil also had no effect on the distribution of functional Canadian Cardiovascular Society grading of angina at the end of the study, and a similar number of patients in both the treatment and placebo groups experienced a deterioration in their angina symptoms during the study.
Multi-centre observational data from a total of 2,558 patients treated with nicorandil and controls subjected to propensity score matching from the Japanese Coronary Artery Disease (JCAD) study provide additional evidence that nicorandil might confer a degree of long-term cardioprotection for patients with stable angina; this study demonstrated a 35 % reduction in all-cause mortality (HR: 0.65; p=0.0008) and 56 % reduction in cardiac death (HR: 0.44; p<0.0001) in patients treated with nicorandil over an average 2.7 years. Among the proposed cardioprotective mechanisms for nicorandil include K+ATP channel activation of myocardial mitochondrial ischaemic preconditioning,35,36 protection against long-term endothelial dysfunction,37,38 stabilisation of atherosclerotic plaques39 and other ancillary properties, including antiplatelet effects.40
Several studies have indicated that nicorandil confers possible beneficial effects after an MI, including the prevention of ischaemic reperfusion injury and microvascular dysfunction during percutaneous intervention,41,42 as well as improvement of myocardial salvage and reduction in mortality following hospital discharge.43,44 There is also some evidence that nicorandil reduces the arrhythmic burden in patients with unstable angina. In the Clinical European Studies in Angina and Revascularisation (CESAR) 2 trial, which included 188 patients with unstable angina, a lower incidence of transient myocardial ischaemia (12.4 % versus 21.2 % p=0.0028), non-sustained ventricular tachycardia (three runs versus 31 runs, p<0.0001), and supraventricular tachycardia (four runs versus 15 runs, p=0.017) was observed during continuous 48 hours ECG monitoring in patients randomised to nicorandil compared to placebo.45
Side-Effects and Drug Cautions
Nicorandil is well tolerated by most patients, with a satisfactory safety profile confirmed by real-world data from 13,260 patients in the Nicorandil Prescription Event Monitoring (PEM) study.46 Other studies have shown that fewer than 10 % of patients report side-effects after treatment with nicorandil for 30 days47 and around 70 % of patients continue to take the medication after 1 year.48
Headache is the most common side-effect of nicorandil, occurring in about 30 % of patients. Other common side-effects include dizziness, flushing, malaise and gastrointestinal upset. Unlike nitrates, the long-term use of nicorandil does not appear to cause significant drug tolerance or rebound angina.49 However, in one study, attenuation of the anti-ischaemic effect of nicorandil was observed after 2 weeks of therapy in terms of time-to-1 mm ST segment depression on exercise testing.50
Nicorandil is avoided in patients with low systemic blood pressure, e.g. due to decompensated heart failure or cardiogenic shock, and contraindicated by concomitant use of phosphodiesterase (PDE)-5 inhibitors (e.g. sildenafil) because of a risk of severe hypotension resulting from this dangerous drug combination. Additional contraindications to nicorandil are detailed in the SPC (https://www.medicines.org.uk/emc/product/652/smpc). Rarely, nicorandil can cause gastrointestinal, skin, mucosal or eye ulceration.51,52 Nicorandil should be stopped immediately if ulceration occurs. Because of the risk of gastrointestinal ulceration, caution is advised when prescribing nicorandil for patients who are also taking corticosteroids. The manufacturer states that gastrointestinal ulcers can progress to perforation, haemorrhage, fistula or abscess; patients with diverticular disease might be at higher risk of these severe complications. Ulcers caused by nicorandil are refractory to conventional ulcer treatment, including surgery, and most only respond to withdrawal of nicorandil therapy. The effects of nicorandil during pregnancy, breastfeeding and on fertility have not been studied in humans. Nicorandil should be avoided in pregnancy and is not recommended during breastfeeding..
Long-acting Nitrates
Nitrates have been used to treat symptoms of chronic stable angina for more than 135 years. Long-acting nitrate vasodilators, including isosorbide mononitrate (ISMN) and isosorbide dinitrate (ISDN), belong to a group of organic nitrate esters with a nitrooxy (-O-NO2) moiety, which act as NO donors.53 Pentaerythrityl tetranitrate is a high-potency long-acting nitrate, which is not currently recommended due to lack of clinical efficacy data.54 Unlike high-potency short-acting glyceryltrinitrate (GTN), bioactivation of ISDN and ISMN appears to be independent of mitochondrial aldehyde-dehydrogenase (ALDH)-2 activity, and remains incompletely understood.55
The clinical effects of nitrates are mediated via activation of endogenous NO-cGMP signaling pathways, including cGMP-dependent kinases and cyclic nucleotide-gated ion channels that reduce intracellular free Ca2+ and desensitise vascular smooth muscle cell contractile elements to Ca2+, causing vasorelaxation.56-58 The action of nitrates in some patients with stable angina may compensate for deranged endothelial function.59
At therapeutic doses, nitrates affect venous capacitance vessels predominately, but also dilate large and medium-sized coronary arteries and arterioles of >100 μm.60 Peripheral venous dilatation decreases venous return, lowering left ventricular end-diastolic filling pressure (preload) and volume, thereby decreasing myocardial work and oxygen demands, and indirectly increasing sub-endocardial blood flow. At higher doses, nitrates result in arterial vasodilatation, reducing systemic vascular resistance (afterload) and blood pressure.
Dosage and Pharmacokinetics
The use of extended-release nitrate formulations with an eccentric dosing regimen, which incorporates a nitrate-free interval of at least 8–10 hours, is recommended to prevent the problem of nitrate tolerance.61 A typical starting dose of extended release ISMN is 30–60 mg once daily, which can be uptitrated to 120 mg or a maximum 240 mg, once daily if required. A single dose of extended-release ISMN provides cover for up to 12–14 hours.
While ISDN undergoes extensive first-pass metabolism by the liver resulting in low bioavailability, oral ISMN is completely absorbed and has 100 % bioavailability, leading to a more predictable dose response with less variation in plasma levels than other nitrates.62
When transdermal GTN is used, tolerance can be avoided by interrupting patches with regular nitrate-free breaks.63,64 However, this approach can be associated with “rebound” angina due to nitrate withdrawal, and the “zero-hour” effect resulting in worsened exercise tolerance in the morning before patch application.65 Rebound angina does not occur with long-acting oral nitrates. Pseudo-tolerance can be problematic in patients treated with nitrates owing to neurohormonal activation and increased levels of circulating catecholamines, sodium retention, and intravascular volume expansion.66
Clinical Efficacy Data
Like other anti-anginal drugs, long-acting nitrates have been shown in clinical trials to improve exercise tolerance, time to symptom onset and time to ST-segment depression during exercise testing in patients with stable effort-induced angina. In a meta-analysis of 51 clinical trials including a total of 3,595 patients, nitrate therapy reduced the number of angina episodes by an average 2.45 episodes per week.67 In another double-blind, placebo-controlled study of 313 patients with stable effort-induced angina, exercise tolerance was significantly increased at four and 12 hours after administration of extended release ISMN, with an incremental dose response observed, and without tolerance or rebound angina developing.68
Do Long-term Nitrates Increase Cardiovascular Risk?
Although historically nitrates have been considered to have a neutral effect on prognosis, emerging evidence suggests that long-term nitrate therapy might have a detrimental influence on clinical outcomes because of the development of endothelial dysfunction in some patients who experience nitrate tolerance. Nitrate tolerance occurs after 12–24 hours of continuous therapy, and has been linked to excess free radical formation, among other mechanisms.69,70 Accumulation of free radicals during nitrate therapy is associated with endothelial dysfunction,71 and increased vasoconstrictor sensitivity underlying rebound angina.72,73,74
In an unblinded study of 1,002 patients with healed MI randomised to treatment with nitrates or non-treatment for an average of 18 months, the rate of recurrent coronary events was higher among those treated with nitrates.75 A deleterious effect of long-term nitrate therapy in patients who have had an MI was also observed from an analysis of data from two large observational studies; however, it is unclear whether patients in these cohorts who were prescribed nitrates had more severe angina symptoms (and greater atherosclerotic burden) than the patients who did not receive nitrates.76
Data from studies in patients with chronic vasospastic angina have also demonstrated higher rates of major adverse cardiac events in those treated with long-term nitrates77 and combined therapy with nitrates and nicorandil.78 In contrast, an analysis of the Global Registry of Acute Coronary Events (GRACE), which included 52,693 patients, found that those receiving long-term nitrates who presented with an acute coronary syndrome tended to have less ST-segment elevation and lower cardiac enzyme release than those who were nitrate naïve.79
Among the potential beneficial actions of nitrates that might contribute to the likelihood of more favourable acute clinical outcomes here include inhibition of platelet aggregation and other antithrombotic and anti-inflammatory effects, as well as protection against ischaemic reperfusion injury mediated in part by impaired opening of the mitochondrial permeability transition pore.80,81 However, the observation of divergent patterns of clinical presentation between patients with an acute coronary syndrome who are prescribed long-term nitrates for antecedent stable angina, and those with acute coronary syndrome who were nitrate-naive, might instead reflect differences in underlying atherosclerotic disease processes in these two patient groups. Further clinical studies are needed to determine the effects of nitrate therapy on long-term prognosis.
Side-effects and Contraindications
Headache is the most common side-effect of nitrates. When occurring within the first hour of nitrate administration, headache is usually due to vasodilation and can often be avoided by starting with a low dosage.82 Occurrence of headache usually dissipates after several weeks of therapy, and co-administration of nitrates with aspirin, prescribed for secondary prevention, can also help to reduce this side-effect. However, in some patients, nitrates can also trigger migraine and other more complex types of headache.83 Approximately 10 % of patients are unable to tolerate nitrates due to headache.84
Other common side-effects of nitrates are light-headedness, flushing, orthostatic hypotension and syncope. The risk of orthostatic hypotension and syncope is greater in the elderly because of age-related autonomic dysfunction. Nitrates are contraindicated in patients with hypertrophic cardiomyopathy, and used with caution in those with aortic stenosis because this could worsen the outflow tract gradient. Other absolute contraindications to nitrates are coadministration with PDE-5 inhibitors because of a risk of profound hypotension, and closed-angle glaucoma. Methemoglobinemia is a rare adverse effect that can occur with large nitrate doses. The safety of nitrates in pregnancy and breastfeeding has not been evaluated, so they should be avoided in these circumstances.
Conclusion
Nicorandil and long-acting nitrates are effective drugs for the treatment of chronic stable angina in patients with effort-induced symptoms arising from epicardial coronary artery stenoses, as well as coronary vasospasm and microvascular angina.
The success of any pharmacological angina therapy hinges on selecting the appropriate drug regimen tailored to individual patient factors and the prevailing underlying angina mechanism(s). Vasodilator drugs, such as nicorandil and long-acting nitrates, are most useful in patients who are unaffected by the haemodynamic side effects of these medications, and in those who have contraindications to rate-limiting anti-angina drugs. Further work is needed to better understand the long-term implications of these drugs on cardiovascular risk.







