Cardiovascular disease (CVD) is the most prevalent non-communicable cause of death worldwide.1 The health and economic implications of caring for a growing ageing population with CVD is enormous. Maintaining a “healthy” elderly population that is mobile and independent with a good quality of life is vital. Testosterone is a sex hormone that is predominant in males, being secreted from the testes and adrenal glands, but it also occurs in lower concentrations in females, where it produced in the ovaries. In men, testosterone is primarily involved in sex differentiation and the development of secondary sex characteristics at puberty and it also contributes to spermatogenesis. It is involved in sex drive in both men and women.
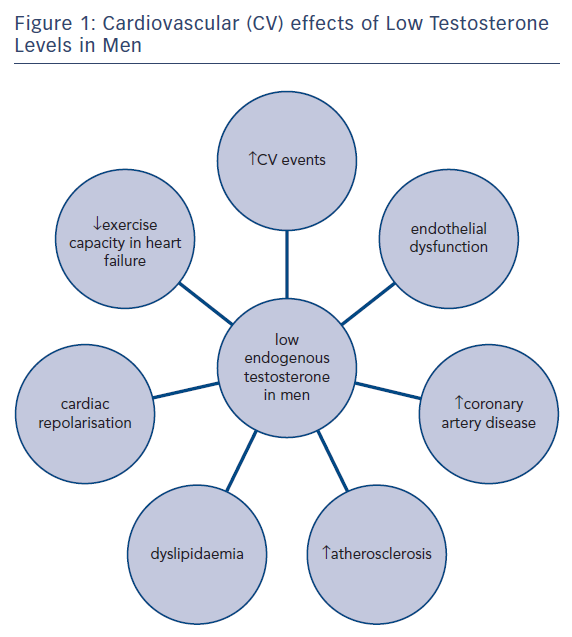
Testosterone has effects directly on the blood vessels of the cardiovascular (CV) system and on the heart, as well as having effects on risk factors for CVD, see Figure 1.2 Serum testosterone concentrations are known to decrease with age. Epidemiological studies indicate that reduced testosterone levels are linked to premature coronary artery disease (CAD),3,4 unfavourable effects on CVD risk factors5–7 and increased risk of CV mortality independent of age.8 A significant number of men with heart failure demonstrate reduced serum testosterone concentrations9,10 and there is early evidence suggesting that low testosterone levels affect cardiac repolarisation.2,11 A recent publication from the Atherosclerosis Risk in Communities observational study reported no association between endogenous testosterone and cerebrovascular disease.12 Any association between endogenous testosterone concentrations and CVD in women has yet to be established. The effects of endogenous testosterone on CVD have been well documented previously.13 Current interest has focussed more on the CV effects of exogenous testosterone, testosterone treatment and its effects on the CV pathophysiology. This has been driven by an increase in testosterone replacement use in men with “low” testosterone concentrations, even without other symptoms of hypogonadism, particularly in North America.14 In the following discussion we will present the current evidence and expert opinions on the CV effects of testosterone treatment, primarily in men as the evidence in women is currently less dependable.
Testosterone and Treatment of CVD
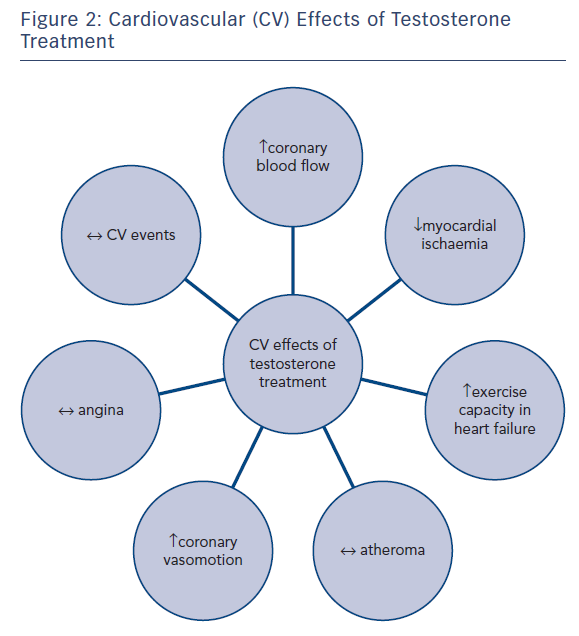
The purpose of testosterone replacement is to normalise low testosterone levels to improve symptoms. Clinically this is advised in patients with symptomatic hypogonadism supported by biochemical evidence; however, many of the studies presented in the following discussion have included men with low testosterone but not necessarily other signs of hypogonadism. Research interest has focussed mainly on the effects of testosterone treatment on the CV conditions of coronary atherosclerosis, myocardial ischaemia, heart failure and, more recently, in cardiac electrophysiology, see Figure 2.
Metabolic Effects of Testosterone Treatment
Endogenous testosterone concentrations are generally inversely related to CVD risk factors in men. Modifiable metabolic risk factors that are affected by testosterone concentrations include dyslipidaemia, central adiposity, insulin resistance and type 2 diabetes (T2DM). Epidemiological and observational studies largely show that low endogenous testosterone concentrations are independently associated with an atherogenic lipid profile, the metabolic syndrome and T2DM.15
Lipid Profile
The evidence to date indicates that testosterone treatment reduces total and LDL-cholesterol concentrations.16–18 The evidence is less clear for HDL-cholesterol and may depend on the route of administration and resultant plasma concentrations of testosterone.15 Triglycerides are generally not changed by testosterone treatment.19 The Testosterone Replacement in Hypogonadal Men with Type 2 Diabetes and/or Metabolic Syndrome (TIMES2) study showed a reduction in lipoprotein(a) in hypogonadal men with and without T2DM or/and the metabolic syndrome treated with testosterone gel.16 In contrast, high doses of testosterone or anabolic steroids dramatically derange lipid profile.20
Dysglycaemia and Diabetes
The evidence consistently shows an association between low testosterone concentrations in men and insulin resistance, metabolic syndrome and T2DM.15 A number of interventional studies show an improvement in insulin resistance and glycaemic control in men with T2DM taking testosterone treatment.16,21,22 In the European TIMES2 study, testosterone gel reduced measures of insulin resistance by up 16 % after 1 year in men with the metabolic syndrome with or without T2DM.16 The BLAST study reported a decrease in HbA1c levels in men with diabetes assigned to testosterone undecanoate for 24 weeks, with a greater effect seen in men with poorly-controlled T2DM.23
Testosterone Treatment and Atherosclerosis
Observational studies indicate that low testosterone concentrations have a detrimental effect on atherosclerosis. For example, testosterone concentrations are negatively associated with carotid artery intimamedia thickness, a measure of atheroma burden.24 The long-term consequences of testosterone supplementation on atherosclerosis in older men have been the focus of a number of recent studies. The Testosterone’s Effects on Atherosclerosis Progression in Aging Men (TEAAM) trial was designed to determine the effect of increasing circulating testosterone concentrations into a range that is mid-normal for young men on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels, but not necessarily with symptoms of hypogonadism.25 The TEAAM trial enrolled just over 300 men in a randomised, placebo-controlled study of testosterone gel. This trial failed to show any benefit of testosterone compared with placebo on carotid intima-media thickness and coronary calcium, which were the co-primary endpoints, nor on the secondary outcomes of sexual function or quality of life. The authors commented that the results may have been influenced by concomitant medications such as statins and that the results of measures of sexual function may differ in men with symptomatic hypogonadism. Following on from TEAAM, the Cardiovascular Trial, one of the seven placebo-controlled Testosterone Trials,26 investigated the effects on coronary atherosclerosis of 1 year of testosterone gel treatment in 138 older, symptomatic, hypogonadal men, assessed by coronary computed tomographic angiography.27 Up to 50 % of this study population had CAD or risk factors for CAD.27 Compared with placebo, testosterone treatment was associated with a significantly greater increase in coronary artery non-calcified plaque volume and total plaque volume. Similar to the TEAAM trial, there was no significant effect on coronary calcium score. An increase in atheroma burden does not necessarily translate into an increased rate of CV events, and neither study was powered to draw conclusions on this. These studies provide evidence for caution in the use of testosterone treatment. Larger studies are needed to understand the clinical implications of these findings.
Coronary Blood Flow, Myocardial Ischaemia and Angina in Men with CAD
Testosterone has direct effects on arteries independent of the reproductive system. Evidence for this has come from both basic scientific28–30 and clinical studies.31–36 Clinical studies show the favourable effects of short- or longer-term exposure to testosterone treatment on coronary and peripheral vasomotion and peripheral arterial stiffness.31–36 Physiological concentrations of intracoronary testosterone cause epicardial coronary artery dilatation and increases in volume blood flow in men with CAD.31 To investigate the effects of longer-term testosterone treatment on coronary blood flow, cardiac magnetic resonance myocardial perfusion scans were performed in men with CAD before and 8 weeks following oral testosterone treatment in a placebo-controlled, cross-over study.32 Myocardial perfusion was enhanced in myocardium supplied by unobstructed coronary arteries but not in areas supplied by coronary arteries with significant atherosclerosis, concurring with previous findings.31,32 The lack of effect of testosterone on myocardium supplied by significantly diseased coronary arteries may explain the results of studies of oral, transdermal and intramuscular testosterone treatment that failed to demonstrate an improvement in the frequency of anginal symptoms of men with CAD.32,37,38 However, these results do not explain the favourable effects of short- and longer-term testosterone treatment on signs of myocardial ischaemia in men with chronic stable angina.37,39,40 The number of studies that have investigated this is small, each enrolling a relatively small number of patients and using varying preparations of testosterone. The conclusions that can be drawn from these data and used to direct clinical practice are therefore limited. The largest study to date included 46 men with stable angina and baseline testosterone concentrations at the lower end of the normal range who were treated with a transdermal testosterone patch or placebo for 12 weeks in a parallel designed study.37 Time to exercise-induced myocardial ischaemia was prolonged by testosterone treatment compared with placebo, with the effect being more pronounced in men with lower baseline testosterone concentrations.37 The study of longest duration thus far is a 12-month parallel, placebo-controlled trial using a testosterone undecanoate injection administered four times per year.40 Thirteen men with angina, signs of myocardial ischaemia on treadmill exercise testing and plasma testosterone concentrations <12 nmol/l completed the study, with the results showing a significant delay in time to myocardial ischaemia in men taking testosterone versus placebo.40 Larger studies are needed to clarify any clinical application of testosterone treatment in men with myocardial ischaemia, anginal symptoms and low testosterone concentrations.
Testosterone and Heart Failure
Testosterone deficiency is estimated to affect approximately a third of men with chronic heart failure. Low circulating levels of testosterone independently correlate with exercise intolerance in men with heart failure41 and relate to the muscle-wasting characteristic of the condition.42 The excess of catabolic hormones and a relative deficiency of anabolic hormones in heart failure have been well documented,42,43 and in the past decade testosterone has been investigated as a potential therapy to correct the anabolic balance.
The first study was a small randomised, double-blind, placebocontrolled, cross-over trial that included men with chronic heart failure.44 Compared with placebo, a single dose of buccal testosterone increased cardiac output and reduced systemic vascular resistance, with the maximal effect coinciding with maximal free testosterone concentrations at 180 minutes.44 Since then, the same group and others have shown the beneficial effects of a variety of testosterone preparations on exercise capacity, oxygen consumption and functional class, with treatment periods lasting from 12 weeks to 12 months.45–47
There has been one study of testosterone treatment undertaken in elderly women with heart failure.48 Given via a transdermal patch for 6 months, testosterone significantly increased the distance walked in a 6-minute walk test and improved peak oxygen consumption, despite the women having pre-treatment testosterone concentrations in the normal range.48 The authors speculated that a peripheral mechanism of action might explain this effect.
A meta-analysis published in 2012 reinforced the beneficial effect of testosterone treatment on exercise capacity and oxygen consumption in patients with heart failure.49 However, reports of the effects of testosterone treatment on muscle strength in men with heart failure are not consistent, with some showing a null effect45 and others finding a benefit.46 This may be because none of these studies included patients with cachexia or muscle wasting. Apart from the testosterone patch,45 generally testosterone was well tolerated in heart failure studies to date. However, on average these studies only lasted a few months and the numbers included were small. Future studies with a longer duration of treatment, a larger study population and including patients with muscle wasting should produce valuable evidence with which to guide clinical practice.
Testosterone and Cardiovascular Events
Controversy surrounds the conflicting evidence on the effects of testosterone on CV events. Prospective population studies, such as the Health in Men Study, found that low testosterone concentrations were associated with an increased risk of incident stroke, but other studies reported no association between testosterone levels and CV events. 50–53 Data from a more recent study in Sweden show an inverse association between both serum testosterone and sex hormone binding globulin concentrations and the risk of CV events, with high testosterone (≥550 ng/dl) predicting reduced fatal and nonfatal CV events.54
Whether testosterone treatment has a favourable effect on CV events in men is a contentious issue. Two large observational studies reported an increased risk of adverse outcomes (death, myocardial infarction and stroke) in testosterone-treated men, particularly in men aged >75 years.55,56 However, the results of these studies have been criticised and must be considered in view of the fact that there was no check of testosterone levels or drug compliance following testosterone initiation.9,57 Contrary to these studies, others have shown a decreased risk of CV events and mortality in testosterone users versus non-users.58,59 An interesting approach was used in an observational study that grouped a large cohort of men with low testosterone concentrations, newly treated with testosterone and then followed for 6 years, into those that achieved a normalised testosterone concentration and those that did not.60 Compared with those not achieving normal testosterone concentrations and with never-users of testosterone treatment, men achieving normalised testosterone concentrations had a lower relative risk of all-cause mortality, myocardial infarction and stroke.60 The discrepancies in the findings of these observational studies may be explained by differences in the age of the study cohorts, co-existing disease (Vigen et al. included men referred for coronary angiography;46 the population studied by Sharma et al. was healthy with no known CVD50) and knowledge of compliance with testosterone therapy after initiation.56,60
Randomised controlled trials of testosterone treatment in men with low testosterone levels also report conflicting results. The Testosterone Treatment in Older Men with Mobility Limitations (TOM) trial of men aged ≥65 years was stopped early due to CV events in the testosteronetreated group;61 however, the study was not designed to report on safety and whether the events were hard endpoints has been a subject of contention.9 Other randomised trials have been too small to draw conclusions about the risks of testosterone treatment, although the reported adverse event rates were similar in the testosteronetreated group versus placebo.25,27,62 There are obvious parallels with hormone-replacement treatment in women in past decades, where observational studies indicated a benefit of postmenopausal hormone therapy on CVD risk but the results of randomised trials did not support this. Unequivocal trials of testosterone treatment in a clinically-relevant population are needed to inform users and prescribers of the safety of testosterone treatment; however, we must acknowledge that this would be an enormous undertaking.63
Risks of Testosterone Treatment
Testosterone administration in hypogonadal men can cause sideeffects such as erythrocytosis (more so with oral preparations), fluid retention, benign prostatic hyperplasia, hepatotoxic and neoplastic effects (mostly with oral preparations but not testosterone undecanoate), gynaecomastia and acne and skin reactions (mostly with patches).64 Increased haematocrit and haemoglobin levels and small detrimental effects on lipids have also been documented. There is much controversy over the association of testosterone treatment with an increased incidence of prostate cancer, which is discussed in detail elsewhere.64,65 In brief, there is no evidence for an increased risk of prostate-cancer-specific death related to testosterone treatment in either direction; however, there is evidence implying that testosterone treatment may adversely affect the progression of prostate cancer.66 Large-scale, long-term trials are needed to clarify the safety of testosterone treatment. The current recommendation from the Endocrine Society is to make “a careful diagnosis of androgen deficiency only in men with consistent symptoms and signs and unequivocally low serum testosterone levels”, then to treat men with confirmed symptomatic androgen deficiency with testosterone therapy.67 Once testosterone treatment is initiated, it is suggested that mid-normal range testosterone levels are aimed for and that therapy is monitored using a standardised plan.67
Conclusions
Low endogenous testosterone concentrations can affect risk factors for CVD, atheroma development and vasomotion beyond the blood vessels of the reproductive system. They are associated with coronary atherosclerosis and heart failure in approximately a third of men. The discrepant results of clinical studies of testosterone replacement in older men with hypogonadism have resulted in controversy over its clinical use, largely concerning variances in CV event rate and the ability of these studies to generate reliable data on this endpoint. Testosterone treatment is not without sideeffects and risks. The Endocrine Society 2010 clinical practice guideline and the European Menopause and Andropause Society give detailed recommendations on the use of testosterone therapy in men with androgen deficiency, supporting such treatment in men with symptomatic androgen deficiency (repeated early morning low serum testosterone levels) followed by scheduled testing for efficacy and adverse events.67,68 Testosterone treatment is not recommended in men with breast or prostate cancer, suspected or increased risk of prostate cancer, haematocrit >50 % or uncontrolled heart failure.67,68 Individualised management of patients is recommended, including the evaluation of comorbidities and careful risk–benefit assessment, and not general prescription of testosterone therapy to all ageing men with low testosterone.67,68 A number of professional organisations agree that there is no consistent evidence that testosterone treatment either increases or decreases CV risk.68 Large studies with hard CV endpoints in older symptomatic hypogonadal men are needed encompassing longer-term treatment periods and investigating the differing types of testosterone replacement available in order to provide strong evidence on which to base clinical decisions in this population.