Rheumatoid arthritis, the spondyloarthropathies, systemic lupus erythematosus, systemic vasculitides, inflammatory myopathies, systemic sclerosis and mixed connective tissue disease are autoimmune rheumatic diseases (ARDs) with high incidence of cardiovascular disease (CVD).1 CVD is usually underestimated in patients with rheumatic diseases, because the main focus of rheumatologists is the signs and symptoms of the systemic disease. Although targeted treatment has significantly contributed to the decrease in disease-related mortality, life expectancy in people with ARDs remains lower compared to the general population, mainly due to increased cardiovascular involvement.2–7
CVD in ARDs is the result of various pathophysiologic mechanisms including systemic, myocardial, vascular inflammation, macro- and micro-vascular ischemia, abnormal coronary vaso-reactivity and diffuse or replacement myocardial fibrosis.8,9 Irrespective of pathophysiologic background, the symptoms of heart involvement in ARDs are subtle and usually underestimated because they are attributed to the underlying systemic disease. However, the development of clinically overt cardiac signs indicates advanced cardiac disease and carries a poor prognosis.10
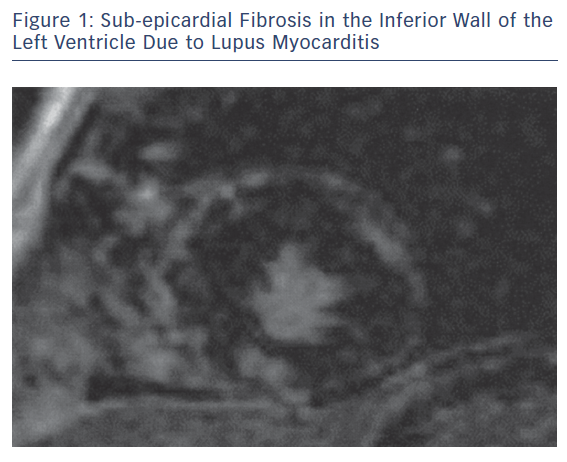
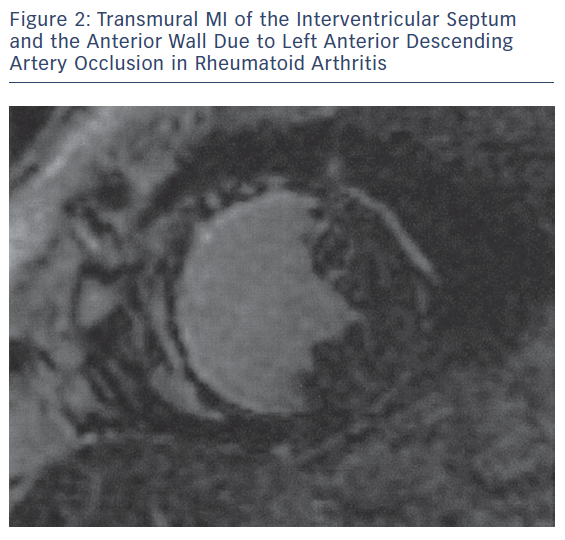
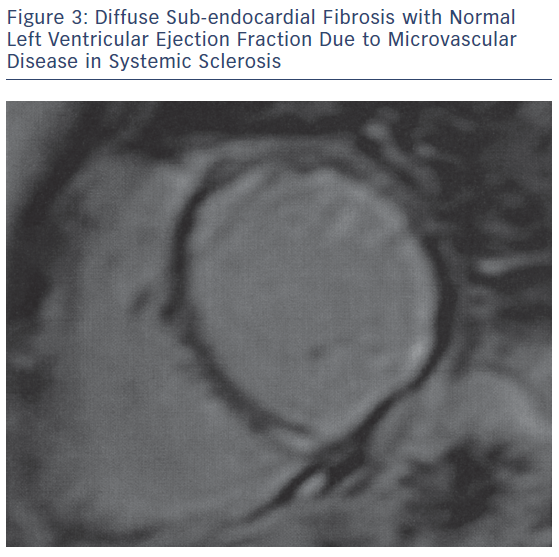
The main pathophysiologic phenomena that need to be assessed as early as possible during the course of ARDs include myocardial and vascular inflammation; macro- and micro-vascular vasculopathy; small epicardial, intra-myocardial and/or sub-endocardial fibrosis, due to inflammation and/or MI (Figures 1–3); acuity of heart involvement; angiography of the great vessels; and assessment of the arterial wall inflammatory process.11–15 These cannot be assessed by the currently used imaging modalities. It should be considered that most ARD patients are female and may be unable to exercise, due to arthritis or muscular discomfort or weakness and therefore the assessment of myocardial ischemia may be problematic.
The Need for a Cardio-rheumatology Outpatient Clinic
The multifaceted presentation of CVD in ARDs created the need for a specific outpatient cardio-rheumatic clinic. Our hospital was among the first in the world to create a dedicated clinic for early diagnosis, management and follow-up of CVD in ARDs. Our outpatient cardio-rheumatic clinic works in close collaboration with hospitals that diagnose and treat ARD patients.
Clinical examination, rest ECG, exercise ECG, echocardiography, nuclear techniques and cardiac catheterisation, if needed, are used as diagnostic tools. The exercise electrocardiogram is commonly used first line for diagnosing myocardial ischaemia. It is widely available and inexpensive, but has low sensitivity in women and is not diagnostic in patients with rhythm disturbances.16 The current American Heart Association/American College of Cardiology guidelines recommend the exercise electrocardiogram as the first-line test for known or suspected coronary disease, but only when the patient is able to exercise. However, ARD patients with musculoskeletal impairment are unable to exercise. The exercise electrocardiogram is also less accurate than alternative stress imaging technologies.17
Transthoracic echocardiography (TTE) is currently the cornerstone of diagnosing CVD. Stress echocardiography combines TTE with a physical, pharmacological or electrical stress, inducing higher pulse rate. If coronary stenosis is present, ischaemia of the left ventricular wall may occur, resulting in a transient worsening of left ventricular wall contractility. Stress echocardiography may provide similar diagnostic and prognostic accuracy with radionuclide stress perfusion imaging, but at a substantially lower cost, without environmental pollution, and with no bio-effects for the patient and the physician. However, it has the limitation of being an operator and acoustic window dependent technique.
Stress myocardial perfusion scintigraphy (MPS) is a useful non-invasive imaging modality for diagnosing patients with suspected coronary artery disease.18,19 However, has serious limitations, including high radiation exposure, imaging artefacts and low spatial resolution that does not allow the detection of small areas of myocardial ischemia and scars.20 It became clear that all the above-mentioned modalities, although useful in cardiology, were unable to diagnose the multifaceted cardiac involvement in asymptomatic ARD patients.21
This discrepancy motivated the application of cardiovascular magnetic resonance (CMR). Compared with other non-invasive imaging modalities, CMR has the advantage of high spatial resolution capable of assessing slight tissue changes occurring during the course of ARDs. Additionally, CMR is versatile and can be used to perform functional, stress-rest perfusion, replacement and diffuse fibrosis assessment, all in one examination, without the use of ionising radiation.21 Furthermore, it can offer evaluation of the patency of the great, peripheral and coronary vessels. Recently, the use of mapping techniques and extracellular volume fraction has allowed the assessment of myocardial oedema and diffuse fibrosis in patients with renal impairment, which is commonly found in ARDs.21
The superiority of CMR in the diagnosis of CVD in ARDs against the other non-invasive imaging modalities is based on the assessment of:
- myocardial ischaemia and/or sub-endocardial/transmural replacement or diffuse fibrosis, due to either macro- or micro-vascular coronary artery disease;22–26
- disease acuity, due to macro- or micro-vascular coronary artery disease;22–27
- extent and disease acuity of myocardial/vascular inflammation;27,28 and
- aetiology of silent/overt heart failure or rhythm disturbances.29,30
The disadvantages of CMR are that it is contraindicated in patients with non-MRI compatible devices or metallic clips and in people with claustrophobia. However, all coronary artery stents, currently implanted valves and MRI-conditioned devices can be safely scanned. In patients with renal failure, the use of gadolinium is contraindicated, due to the risk of nephrogenic fibrosis. In these patients, we can apply non-contrast imaging protocols and perform both function and tissue characterisation evaluation. Additionally, CMR performs less well in patients with severe arrhythmia and who are unable to hold their breath.21
Role of CMR in the Evaluation of Anti-rheumatic and Cardiac Treatment
There are only a few studies supporting a role for CMR in the evaluation of anti-rheumatic and cardiac treatment in ARDs. A previous study by our group documented that CMR can detect early silent cardiovascular (CV) lesions, assess disease acuity and successfully evaluate the effect of both cardiac and anti-rheumatic medication on the CV system.31
In another study, the CMR findings of 246 ARD patients with typical cardiac symptoms (n=146) or atypical cardiac symptoms (n=100) were retrospectively evaluated. CMR in symptomatic ARD patients with normal echocardiographic findings assessed disease acuity and identified vasculitis, myocarditis and MI that influenced the CV risk stratification of ARD patients.11 Furthermore, occult CMR lesions, including myocardial oedema, myocarditis, diffuse sub-endocardial fibrosis and MI, were not unusual in treating naïve ARDs and may be reversed with appropriate treatment.32 Additionally, stress CMR has successfully detected silent myocardial Raynaud phenomena in patients with ARDs and known peripheral Raynaud phenomena, and thus motivated the early start of relevant cardiac treatment.33
CMR offers the potential to identify ARD patients at high risk of ventricular tachycardia or VF, thus influencing both cardiac and anti-rheumatic treatment and possibly modifying the criteria for ICD implantation.34 Although the role of cardiac treatment is established for early morphologic or functional cardiac changes,35 clear guidelines for anti-rheumatic treatment are still missing.
According to our experience, a baseline study, including clinical, ECG and TTE evaluation, should be performed at diagnosis of ARDs and a CMR should then be recommended if:
- there is a mismatch between clinical findings and imaging/laboratory findings;
- there is new onset heart failure;
- if there is any kind of arrhythmia;
- if the rheumatologist plans to change treatment, especially if there is a plan to initiate biologic agents;
- if there is any increase in troponin, brain natriuretic peptide or D-dimers, even if there are only subtle symptoms;
- if the patient is being treated with hydroxyl-chloroquine or biologic agents; or
- if the patient notes any kind of typical or atypical cardiac symptoms and the routine cardiac evaluation is normal.
Conclusion
CMR allows the early diagnosis of various CV pathophysiologic phenomena in ARDs. Preliminary studies suggest it has a promising role in prompting modifications of anti-rheumatic and cardiac treatment in ARDs with CVD. The diagnostic potential of CMR strongly supports the view that we should “save the last dance” for CMR in early diagnosis, risk stratification and treatment follow-up of CVD in ARDs.