“When the cause of the sickness is unknown, only a miracle can cure it.” Don Quixote, Miguel de Cervantes, 1605.
The coronavirus 2019 (COVID-19) pandemic was caused by the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1,2 COVID-19 rapidly spread from China to most countries around the world, and on 11 March 2020 the WHO declared a global pandemic. The COVID-19 pandemic has already had a major negative impact on global health systems and the economy.3 From a pharmacological point of view, the rational treatment of a disease is based on the knowledge of its pathophysiology, the identification of a therapeutic target and trial confirmation of the effectiveness of the intervention. Indeed, under normal circumstances, well-designed randomised controlled trials (RCTs) are key to confirm that a pharmacological intervention directed at a selected target is effective and safe. However, these are not normal times and we find ourselves facing a pandemic caused by a virus of which we know very little, and for which there is no clear understanding of the pathogenesis or pathophysiology of the diseases triggered by COVID-19. Moreover, little is known regarding the short- and long-term clinical consequences of the disease and why different individuals respond so differently to the aggression of the virus. Without specific effective therapies for COVID-19 we are facing a very large death toll and the collapse of many health services worldwide.
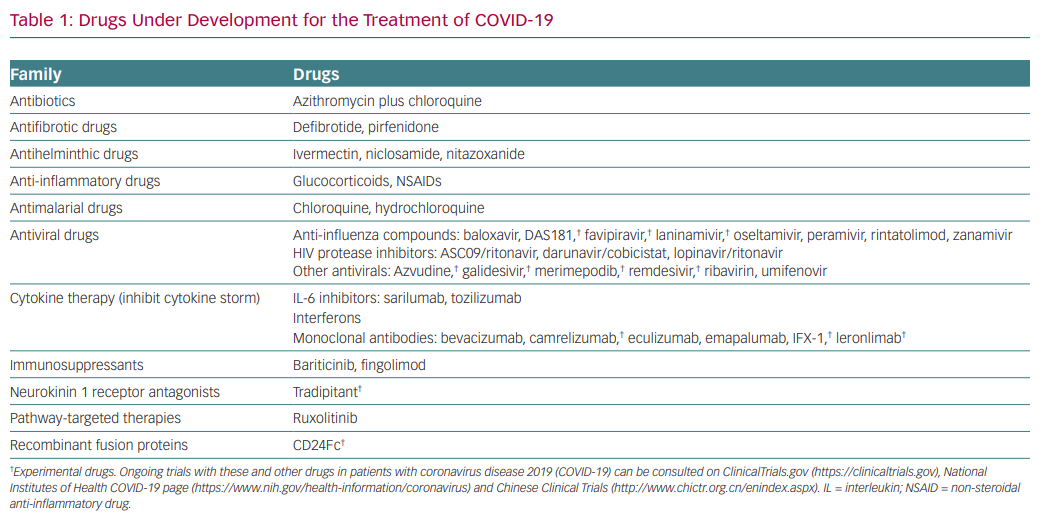
As a result of the severity of the problem, in the past few months numerous drugs have been proposed that might be effective in the treatment of COVID-19 (Table 1). However, due to the lack of true scientific data to support a therapeutic strategy for COVID-19, it has become apparent that we truly are shooting in the dark. This article aims to convey my reflections on the current situation from a clinical pharmacologist’s viewpoint. Several issues have attracted my attention since the initial reports emerged from China at the time of rapid death progression in that country.
The Ibuprofen Story
On 14 March 2020 the French Health Minister, Olivier Véran, claimed on Twitter that anti-inflammatory drugs such as ibuprofen or cortisone could be an aggravating factor in people with COVID-19.4 A few days later, on 18 March, the WHO recommended using paracetamol instead of ibuprofen. On 19 March, the WHO updated its advice on the official Twitter account: “Based on currently available information, WHO does not recommend against the use of ibuprofen”.5 Finally, on 14 April, the Commission on Human Medicines Expert Working Group on COVID-19 concluded that there is currently insufficient evidence to link ibuprofen or other non-steroidal anti-inflammatory drugs, with a susceptibility to contracting COVID-19 or the worsening of its symptoms.6
Use of Renin-Angiotensin-Aldosterone System Inhibitors in COVID-19 Patients
The angiotensin-converting enzyme 2 (ACE2) is the receptor responsible for SARS-CoV entry into cells, and because ACE2 expression increases in patients treated with ACE inhibitors (ACEIs) and angiotensin II type 1 receptor blockers (ARBs), it was hypothesised in a letter published in The Lancet Respiratory Medicine that patients treated with agents capable of increasing ACE2 availability/density such as ACEIs, ARBs, and ibuprofen could be at higher risk of developing severe and fatal COVID-19 infection.7–9 Furthermore, the authors added that ACEIs can modify the adaptive immune response and suggested that long-term use of ACEIs might suppress the adaptive immune response in individuals affected by COVID-19. The article caused great consternation among patients and physicians alike. Although it was somewhat ‘reasonable’ on theoretical grounds, there is no clinical or scientific evidence to indicate that treatment with these important medications should be abandoned or discontinued in patients affected by SARS-CoV-2 infection.10,11
The above examples are of concern, given that they led to confusing and contradictory recommendations, created uncertainty among prescribers and increased mistrust in medical decisions among the general public. They may also fuel the proliferation of false information or imaginative uses for medications or substances that may not only be ineffective but also extremely dangerous and life-threatening.
As expected, scientists all over the world are assessing whether drugs that inhibit SARS-CoV-2 in vitro (even when their effects in vivo may remain uncertain), agents with broad-spectrum antiviral actions, previously developed and approved for the treatment of other viral infections (particularly RNA viruses, i.e. influenza and HIV) or drugs approved for other clinical indications that exhibit potent broad-spectrum antiviral effects (i.e. chloroquine, hydrochloroquine, ivermectin) might also be effective against SARS-CoV-2 (Table 1). Azithromycin was also tested in this context because its early use is known to reduce the likelihood of recurrent severe episodes of lower respiratory tract illness.12 The race to find an effective medication is progressing at high speed, but the risk is that the quality of the research could be compromised by the anxiety driving some of the research projects.
Severe pneumonia caused by human coronavirus is often associated with massive inflammatory cell infiltration, increased plasma concentration of inflammatory cytokines/chemokines and multiorgan failure, resulting in acute lung injury and acute respiratory distress syndrome.13,14 Indeed, high virus titres and deregulated cytokine/chemokine responses cause a cytokine storm, which results in high morbidity and mortality due to immunopathology.1,14,15 Furthermore, a recent meta-analysis found that measurement of circulating interleukin-6 (IL-6) may predict disease progression in COVID-19 patients.16 This suggests that besides controlling viral load, novel strategies directed at attenuating the cytokine storm (e.g. IL-6 receptor antagonists, immunosuppressants, interferons, fingolimod) may possibly improve clinical outcome, although these medications can increase the risk of secondary infection.
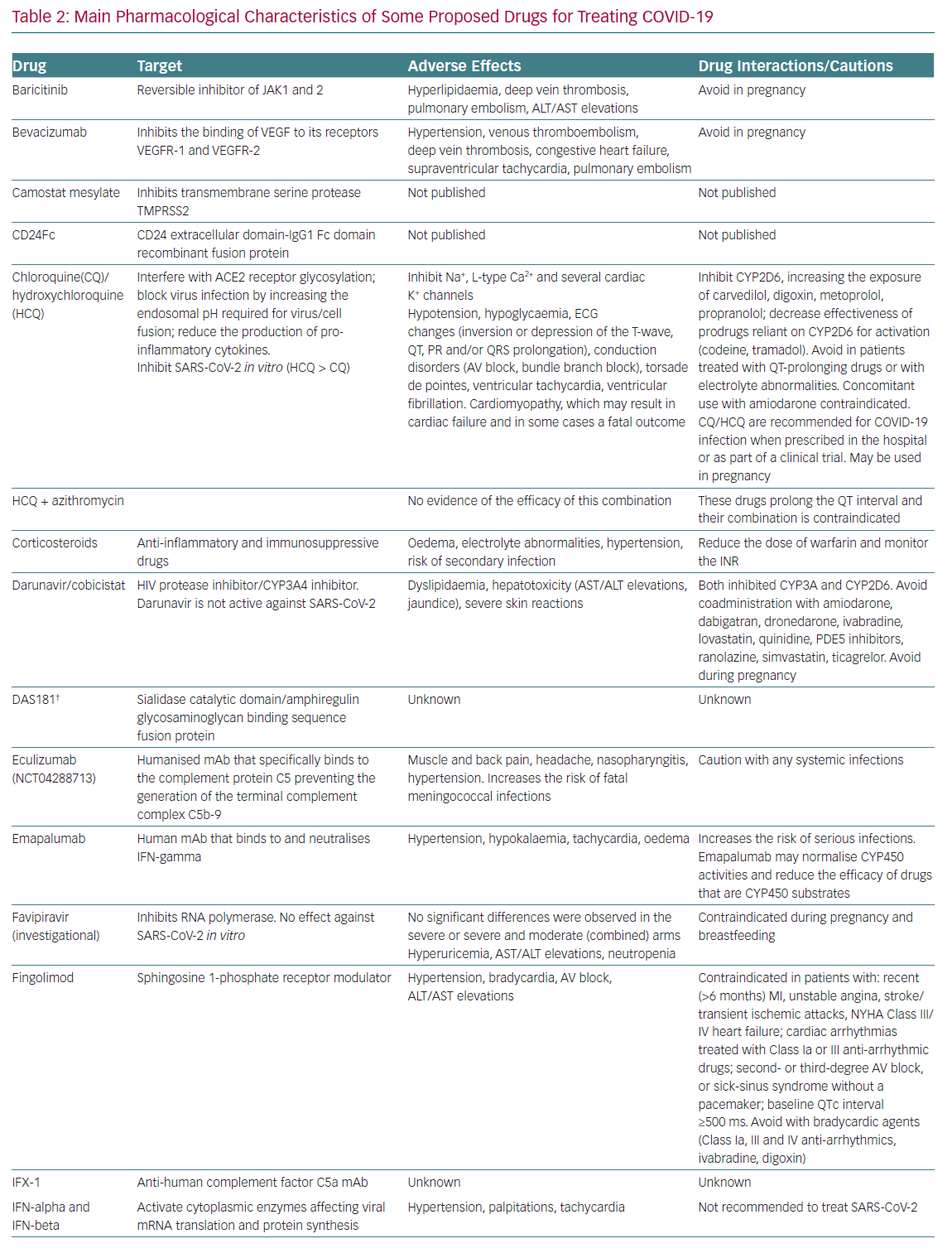
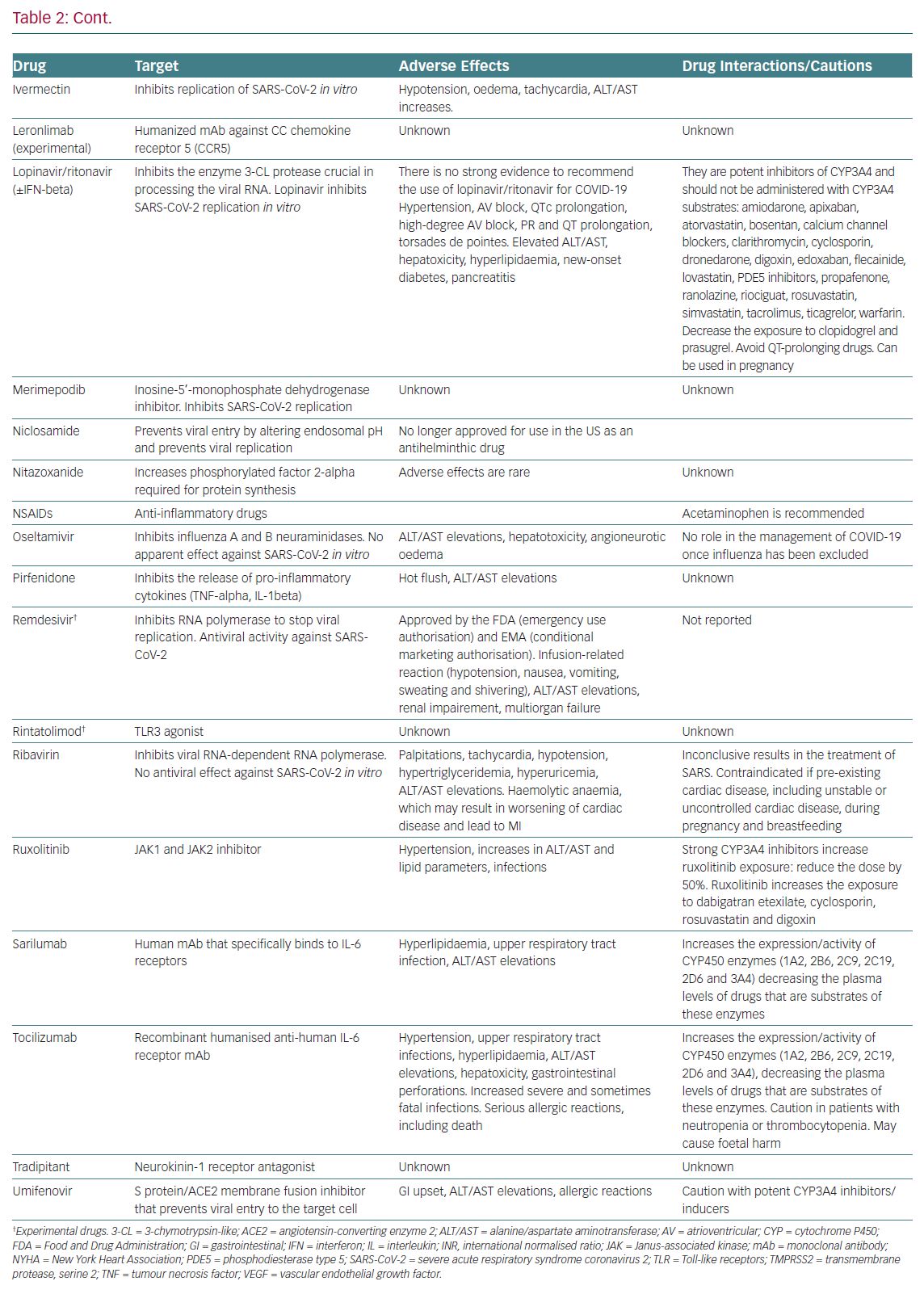
Table 2 summarises the mechanisms of action, the main adverse effects and potential interactions with other cardiovascular drugs,
of drugs currently under clinical investigation for the treatment of COVID-19.
Some Pharmacological Reflections
COVID-19 is a new pandemic and to date, no specific drug (or vaccine) has been found to be effective. Therefore, there is no solid evidence at this point in time that allows treating physicians to use COVID-19-specific treatments, thus management should include the prompt implementation of proven infection prevention and control measures and supportive care, ranging from symptomatic outpatient management to full intensive care support.17 At the onset of the COVID-19 outbreak and in view of the lack of specific treatment for this new coronavirus, SARS-CoV-2, antiviral screening programs identified many antiviral agents that showed in vitro activity against SARS-CoV-2. These drugs are presently being tested in ongoing clinical trials. Several issues have emerged in relation to the strategies that are currently being implemented.
The first is whether the antiviral effects reported in vitro could also be achieved in vivo. Sometimes the concentrations at which these agents inhibit SARS-CoV in vitro are much higher (‘supratherapeutic’) than the therapeutic plasma levels reached at the recommended doses. Thus, it is possible that plasma levels with meaningful activity against SARS-CoV in vitro would not be achieved in vivo without potentially adverse effects. This may be the case for ivermectin and ribavirin. 18 Furthermore, several drugs under development or already approved for other indications, exhibiting a broad-spectrum antiviral activity, are now being tested in COVID-19 patients even when their efficacy and safety in in vivo models or in patients is uncertain. Some antivirals (favipiravir, baloxavir) show no activity against SARS-CoV-2 in vitro at concentrations under 100 μmol/l, but they are, however, being tested in ongoing trials.19 It would not be surprising if the results were disappointing.
The second is how the pharmacokinetic properties of many of the currently investigated drugs, which were studied in healthy volunteers or in patients affected with the disease for which the drug was initially approved, behave in COVID-19 patients.
In the rush to find a treatment, several ongoing trials are being conducted with drugs that were developed but not approved as yet, or were approved to treat other non-COVID-19 diseases (repurposed agents). However, the half maximum effective concentrations and the recommended doses of these drugs against different viruses are quite different and are based on the particular type of infectious disease. Therefore, the question is how will the most effective and safer dose be calculated in patients with COVID-19? In fact, doses of a given drug may differ in different trials. For example, the dose of chloroquine used to treat COVID-19 varies from 500 mg orally twice daily for 5–10 days to a loading dose of 400 mg twice daily for 1 day followed by 200 mg twice daily for 4 days.20–22 Thus, studies are needed to determine the optimal dose range for the drugs used in the treatment of COVID-19 patients.
We must keep in mind that patients with COVID-19 and pre-existing cardiovascular disease have an increased risk of developing severe symptoms and death, and COVID-19 itself is associated with several cardiovascular complications, including acute myocardial injury, myocarditis, arrhythmias and venous thromboembolism.8,23 Some drugs tested in ongoing trials produce serious cardiovascular and non-cardiovascular adverse effects (hepatotoxicity, neutropenia and increased risk of infection), which clearly represents a clinical challenge (Table 2). It is also recommended to avoid the coadministration of drugs that prolong the QT interval (azithromycin with chloroquine/hydroxychloroquine) and to prescribe chloroquine/hydroxychloroquine, which can produce serious cardiovascular events, only in-hospital or as part of a clinical trial, in patients with COVID-19.
Although corticosteroids were not initially recommended for COVID-19 patients because of the lack of proven benefit and potential harm with these drugs, the RECOVERY Collaborative Group has very recently shown that in hospitalised patients with COVID-19, the use of dexamethasone for up to 10 days resulted in lower 28-day mortality than usual care among those who were receiving either invasive mechanical ventilation or oxygen alone at randomisation.24
Interestingly, the safety profile of drugs not already approved by the Food and Drug Administration (FDA) or the European Medicines Agency is based on limited published evidence, so that their safety profile remains uncertain and we must await the results of ongoing prospective, randomised, controlled studies before the widespread adoption of these drugs.25 Additionally, some drugs (HIV protease inhibitors, IL-6 receptor antagonists) inhibit or induce the activity of some cytochrome P450 3A4 enzymes and increase or decrease, respectively, the exposure of multiple drugs that are substrates of such isoforms (Table 2). Therefore, investigational anti-COVID-19 therapeutics should be used only in approved, RCTs.
Almost on a daily basis, case reports, observational or small retrospective, non-randomised studies are published in major journals and non peer-reviewed journals. Albeit describing interesting cases and findings, many of these articles suffer from serious methodological problems, including lack of blinding, small sample size (<50 patients per group) and marked variations in the inclusion/exclusion criteria, presence of background therapy and varied treatment regimens (different doses, different duration of therapy and different routes of administration), which makes it difficult to ascertain the true treatment effect. Furthermore, the effects of current treatments on quality of life and drug safety are underreported. This variability can explain why even studies performed in the same country with the combination of hydroxychloroquine and azithromycin may lead to contradictory results. 26,27 Additionally, the non-standardised collection of clinical data limits the conclusions from retrospective analysis. As an example, one trial recruiting 21 patients concluded that 91% of patients were discharged on average 13.5 days after the treatment with tocilizumab (400 mg IV), with most patients receiving only one dose; however, the lack of a comparator group limits the interpretation of these results.28
Furthermore, sometimes the preliminary exciting results of one study are not confirmed in other trials. This may indeed be the case of remdesivir. In 53 patients hospitalised for severe COVID-19 who were treated with compassionate-use remdesivir, clinical improvement was observed in 68% of patients.29 These promising results led to a Phase III trial in China. Surprisingly, on 23 April 2020, the WHO reported the preliminary results of a Phase III Chinese trial but this report was quickly withdrawn, suggesting that remdesivir did not have beneficial effects in COVID-19 patients or that it was not able reduce the virus in the bloodstream. Interestingly, the researchers responsible for the study indicated that they had not approved its posting. This is a good example of how rushing to obtain results can lead to speculation and unhelpful rumours about the effectiveness of drugs. Nevertheless, on 1 May 2020, the FDA issued an Emergency Use Authorisation for emergency use of remdesivir for the treatment of hospitalised COVID-19 patients.30 Ongoing clinical trials will define which patients will best benefit from remdesivir treatment.
Conclusion
COVID-19 is a new pandemic and, to date, no specific drug has been found to be effective. The identification of effective interventions against COVID-19 (including vaccines) has become a public health priority. Scientists and physicians are facing the challenge to better understand the characteristics of this new virus and the pathophysiology of the disease that it causes, in order to identify possible therapeutic targets and effective therapeutic drugs and vaccines. Despite these efforts, at the present time, we are still shooting in the dark for the reasons mentioned here. Adequately powered randomised clinical trials are needed to establish the efficacy and safety of therapeutic agents prior to the widespread adoption of these therapies. Attempts should be made to develop suitable treatment protocols and to standardise datasets that could facilitate meaningful analysis of treatment effects and clinical outcomes.