Although cardiovascular mortality has declined progressively in developed countries, ischaemic heart disease (IHD) and chronic stable angina cause a worse prognosis and poor quality of life and can dramatically increase healthcare costs.1–4 Traditionally, chronic stable angina has been interpreted as reversible episodes of myocardial ischaemia due to the presence of coronary artery disease. Coronary artery disease hampers coronary blood flow augmentation in response to an increase in myocardial oxygen consumption, thus causing myocardial ischaemia. Based on this assumption, myocardial ischaemia is the direct consequence of an imbalance between the demand and supply of oxygen and metabolites. Current guidelines recommend pharmacological agents that can modulate cardiac work, such as beta-blockers and calcium channel blockers (or modulate coronary blood flow (nitroglycerin), alone or in combination, in addition to percutaneous coronary intervention.1 These strategies were expected to prevent episodes of myocardial ischaemia, improving symptoms and prolonging survival but available data indicates that this approach is not effective and about one-third of patients experience angina despite successful coronary revascularisation, or medical therapy and percutaneous coronary interventions.5–7 The failure of these interventions to improve the prognosis of patients with IHD shows an oversimplified approach to mycoardial ischaemia and its multifactorial aetiology.
Recent research has shown that myocardial ischaemia may be precipitated by several different mechanisms, including coronary stenosis, coronary vasospasm, microvascular dysfunction and mitochondrial dysfunction.8,9 Therefore, a combined approach to IHD that targets multiple mechanisms may be a more successful treatment strategy.1,10 Targeting cardiac myocytes, protecting them from ischaemic damage and modulating myocardial metabolism could improve cardiac efficiency and long-term outcomes.11–13 Indeed, studies have shown that metabolic modulation therapy plays a critical role in the acute phase of ischaemic events, affecting the results of acute interventions and the subsequent development of heart failure (HF), stunned and hybernated myocardium, as well as chronic stable angina.14
Heart Metabolism in Health and During Ischaemia
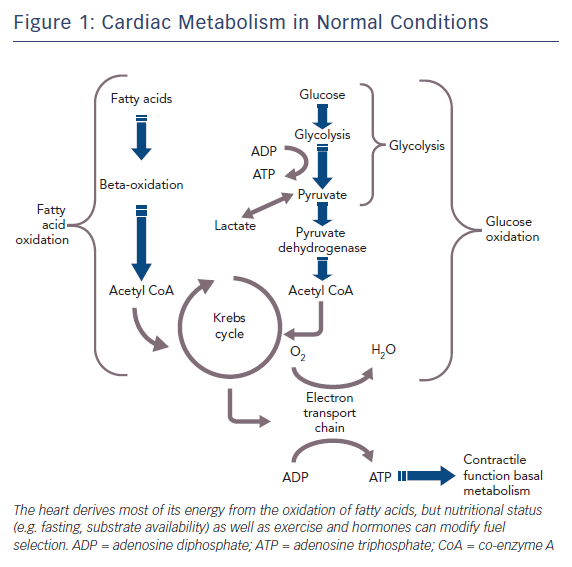
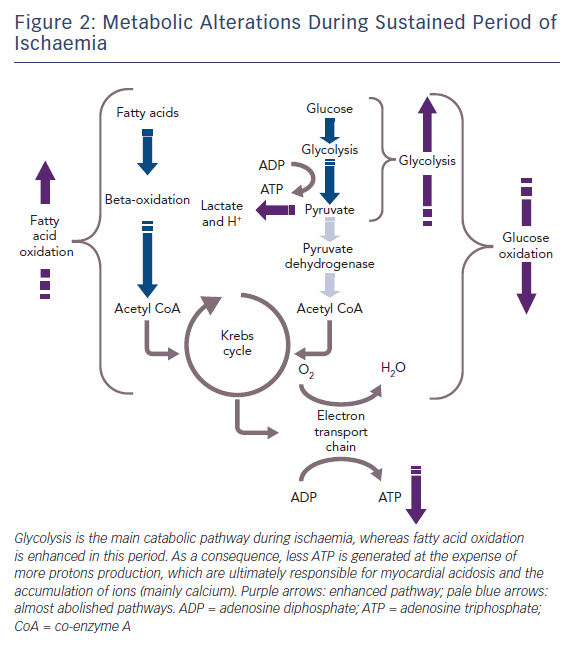
The healthy heart derives most of its energy from the free fatty acid pathway that accounts for about two thirds of energy production in the form of adenosine triphosphate (ATP), and the rest is derived from glucose oxidation and lactate. The healthy heart is able to modulate the use of substrates according to availability, general nutritional status and exercise levels. During mild to moderate cardiac ischaemia, myocardial cells respond by accelerating glucose uptake to generate enough ATP to maintain ionic gradients and calcium homeostasis. Paradoxically, during prolonged and severe ischaemia, the myocardium continues to derive most of its energy from beta-oxidation despite a high rate of lactate production. In this condition, high rates of fatty acid oxidation further inhibit glucose oxidation due to competitive interaction, known as the Randle mechanism.15 Although the complete oxidation of fatty acid produces more ATP than complete oxidation of glucose, a greater amount of oxygen is required. Therefore, for a given amount of oxygen consumed, glucose metabolism is ‘oxygen sparing’ compared with fatty acid metabolism, producing about 15 % more ATP. Therefore, when there is a low availability of oxygen, fatty acid oxidation has an unfavourable effect on cells requiring more oxygen, producing less ATP and more reactive oxygen species (ROS), further depressing mitochondrial respiratory efficiency. These metabolic changes are responsible for metabolic, morphological and functional alteration of the myocardium leading to arrhythmias and contractive failure.16–18 Figure 1 depicts energy generation in the healthy heart, while Figure 2 shows metabolic derangements occurring during ischaemia.
Coupling glycolysis to glucose oxidation is of pivotal importance due to the link between these enzymes and the activity of two survival-promoting membrane-bound pumps, namely the sodium-potassium ATPase, and the calcium uptake pump of the sarcoendoplasmic reticulum (SERCA).19 This interplay explains the efficacy of anti-ischaemic fatty acid inhibitors such as trimetazidine and ranolazine. In line with this, the greatest progress for patients with stable angina came with the use of metabolic therapy, particularly with the advent of direct inhibitors of myocardial fatty acid oxidation, trimetazidine and ranolazine.17
Cardiac Metabolic Modulators
Significant progress has been made since it was acknowledged in 1999 that it was necessary to treat ischaemia, a metabolic disorder, with metabolic therapy.20
Given the interdependence between fatty acid and glucose oxidation, metabolic modulation therapy with optimisation of the use of energy substrate can be achieved by either inhibiting fatty acid oxidation or stimulating glucose oxidation. This can be achieved through three major strategies:
- directly enhancing glucose oxidation;
- decreasing the circulating levels of fatty acids and/or their uptake by cardiac myocytes or mitochondrion; and
- directly inhibiting the enzymes that participate in fatty acid oxidation.
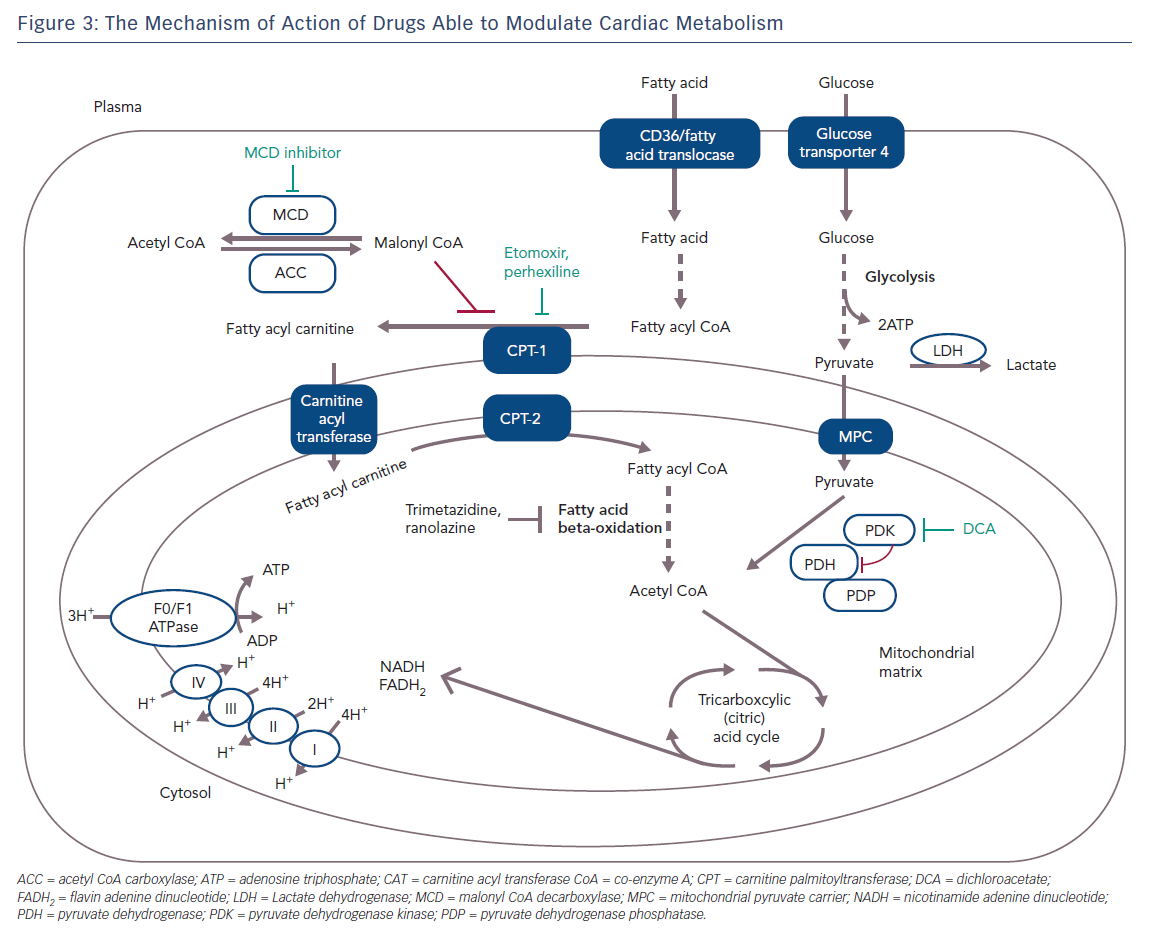
Figure 3 shows the specific mechanism of cardio-metabolic drugs.
Strategies to Enhance Glucose Oxidation
Dicholoroacetate
The rate-limiting step for glucose oxidation is catalysed by the pyruvate dehydrogenase (PDH) complex, which consists of PDH, PDH kinase (PDK), and PDH phosphatase (PDHP) enzymes.21 PDH flux is increased in response to increases in glycolysis and an increased generation of pyruvate, while PDH flux is decreased by increased ratios of mitochondrial nicotinamide adenine dinucleotide (NADH/NAD+) and acetyl-coenzyme A (acetyl CoA/CoA).22 PDHP dephosphorylates activate PDH, whereas PDK phosphorylates inhibit it.21 Dichloroacetate improves glycolysis and glucose oxidation coupling and decreases proton production by inhibiting PDK activity and stimulating mitochondrial PDH.23
There are no data available on the use of this approach on patients with heart disease. Despite the promising experimental evidence when used in other pathological conditions, dichloroacetate treatment has been associated with neurotoxicity which has prevented its use in clinical applications.24
Strategies to Reduce Cellular/Mitochondrial Fatty Acid Uptake
Carnitine Palmitoyl Transferase Inhibitors: Perhexiline
A strategy to inhibit mitochondrial uptake of fatty acids is to suppress the rate-limiting enzyme for the mitochondrial uptake of fatty acids, such as carnitine palmitoyl transferase (CPT) 1 or 2. Perhexiline is a reversible CPT-1 and, to a lesser extent, a CPT-2 inhibitor, and has been shown to relieve angina (Figure 3); it attenuates the increase in diastolic tension associated with myocardial ischaemia, thereby improving myocardial efficiency.25,26 Inhibition of CPT-1/CPT-2 by perhexiline improves the efficiency of myocardial oxygen use by at least 13 %. However, after perhexiline administration, cardiac efficiency increases by about 30 %, suggesting additional mechanisms at work.27 However, perhexiline has been associated with infrequent but serious hepatotoxicity and neuropathy that necessitates regular monitoring of plasma levels and makes perhexiline unsuitable for use in patients with hepatic or renal dysfunction.28
Malonyl CoA Decarboxylase Inhibitors
Malonyl CoA is another potent, endogenous inhibitor of CPT-1 which decreases the uptake of fatty acids into the mitochondria, thereby reducing mitochondrial fatty acid beta-oxidation. Malonyl CoA decarboxylase (MCD) degrades malonyl CoA and this leads to an increase in fatty acid oxidation. Inhibition of MCD significantly increases malonyl CoA levels, therefore causing a significant decrease in fatty acid oxidation rates and a subsequent increase in glucose oxidation rates (Figure 3). Inhibition of MCD in animal models has led to a significant improvement in cardiac functional recovery of aerobically reperfused ischaemic hearts.29 Inhibition of MCD in the heart appears to be a safe and promising therapeutic target for IHD but it is not yet ready for clinical testing.
Strategies to Reduce Fatty Acid Oxidation
The concept of metabolic protection of the ischaemic myocardium is gaining more attention and is supported by clinical studies that have confirmed the beneficial effect of fatty acid oxidation inhibitors such as trimetazidine (TMZ) and ranolazine (RNZ), able to couple glycolysis to glucose oxidation.17
Trimetazidine
Trimetazidine (TMZ) has been the first and, for many years, the only registered drug in this class of anti-anginal agents. The beneficial effect of TMZ as an anti-anginal drug was established before it was discovered that the drug acts via partial inhibition of myocardial fatty acid oxidation.17,30,31 Initial preclinical studies demonstrated that it was cytoprotective in several models of myocardial ischaemia and reperfusion.32 Kantor et al. have shown that TMZ specifically inhibits the long-chain activity of the enzyme acetyl CoA C-acyltransferase; an enzyme that is commonly referred to as 3-KAT. The 3-KAT enzyme catalyses the terminal reaction of fatty acid beta-oxidation, using long-chain 3-ketoacyl CoA as a substrate, to generate acetyl CoA.33 These results suggest that TMZ removes the inhibition on PDH by inhibiting 3-KAT in the mitochondrial matrix, and increases the rate of glucose oxidation (Figure 3). TMZ has been shown to significantly increase the rate of glucose oxidation in rats’ hearts despite only modestly reducing the rate of fatty acid oxidation.33,34 In one study, a single dose of TMZ was shown to increase plasma levels of adenosine, suggesting a preconditioning role for this drug.35–37 Several studies have shown that TMZ alone or in addition to calcium channel blockers can effectively improve symptoms, quality of life and markers of ischaemia on stress tests in patients with chronic stable angina and ischaemic cardiomyopathy when used on top of conventional treatment.38–44 Moreover, clinical trials have proved the efficacy of this metabolic agent in refractory angina, and have supported the superior benefit associated to the addition of this metabolic agent to classic haemodynamic agents, such as beta-blockers or nitroglycerin.45–47
In the TRIMetazidine in POLand (TRIMPOL) II trial, a randomised, double-blind, placebo-controlled, multicentre study that recruited patients with stable angina, the addition of TMZ (20 mg three times a day) or placebo on top of metoprolol, resulted in an improvement in time to ST segment depression on exercise tolerance testing, total exercise workload, mean nitrate consumption and angina frequency when compared with patients receiving placebo.45 Similar efficacy was observed in the subgroup of patients revascularised with recurrent angina. The time to 1 mm ST-segment depression (STD) was increased with TMZ by 80 seconds and was significantly greater than that recorded in the placebo group (p<0.01). The time to onset of angina was significantly greater for the group treated with TMZ in comparison with placebo (p=0.031). The total duration of exercise was significantly greater than that recorded for patients with placebo plus metoprolol (p=0.048). A similarly significant observation was made regarding workload (p=0.035). The maximum ST-segment depression at peak exercise was significantly smaller in the TMZ group than the placebo group (p<0.01). The mean number of angina attacks per week was also reduced in patients receiving TMZ compared with those treated with placebo (p<0.01).45
The efficacy and acceptability of TMZ in combination with haemodynamic agents (beta-blockers or long-acting nitrates) was tested in the Trimetazidine in Angina Combination Therapy (TACT) study.48 After 12 weeks of therapy, exercise test duration significantly increased in the TMZ group, as well as time to 1 mm STD and time to onset of anginal pain. The mean number of angina attacks per week decreased in the TMZ group, along with mean consumption of short-acting nitrates per week. The addition of TMZ on beta-blockers or long-acting nitrates therapy, significantly improved exercise stress test parameters and angina symptoms compared with placebo. In another randomised, double-blind, controlled trial in people with angina who were symptomatic despite taking propranolol, Michaelides et al. demonstrated that the addition of TMZ significantly decreased the mean number of angina attacks twice as much as isosorbide dinitrate alone.49 Similar results were also observed in the Efficacy of Trimetazidine on Functional Capacity in Symptomatic Patients with Stable Exertional Angina (VASCO-angina) study. This was a randomised, double-blind, placebo-controlled trial, which assessed the anti-anginal efficacy and safety of standard and high-dose modified-release TMZ (70 mg per day and 140 mg per day) in symptomatic and asymptomatic patients with chronic IHD receiving 50 mg per day of atenolol, on exercise test parameters.50 The VASCO-angina study confirmed the efficacy and tolerability of standard and high-dose TMZ in improving effort-induced myocardial ischaemia and functional capacity in patients with chronic stable angina receiving background beta-blockers.50 Furthermore, other studies and meta-analysis have supported the use of TMZ to improve clinical manifestation (i.e. total exercise duration, time to 1-mm ST-segment depression, weekly number of angina attacks, weekly nitroglycerin use, quality of life improvement) in patients with stable IHD.51
TMZ has also been proven to be effective in patients with microvascular angina.52 In a recent study, TMZ was given for 3 months (35 mg twice a day) to patients with microvascular angina and it was associated with better control of angina symptoms and stress testing results compared with conventional therapy (beta-blockers/calcium channel blockers, statins, antiplatelets, long-acting nitrates). Moreover, there was an improvement in myocardial perfusion and endothelial function probably due to a reduction in serum endothelin-1 (ET-1) levels and an increase in total antioxidant status.53 There was a collective decrease of average number of attacks per week and silent myocardial ischaemia, a reduction of mean weekly consumption of short-acting nitrates, an improvement in quality of life, reduced severity of main clinical manifestations of chronic heart failure (CHF) and lowering of its functional class.50,54–58 Moreover, similar efficacy of TMZ has been demonstrated in both men and women and it is also suitable for people with diabetes.59–62 A single dose of 60 mg TMZ (the normal cumulative daily dose) has shown to improve exercise capacity in people with angina pectoris, as reflected by an increase in the duration of exercise, total work performed, and an improvement in ECG signs of ischaemia. All these effects occur without any detectable chronotropic or vasomotor effect.63 One ongoing large, international, randomised study – the efficAcy and safety of Trimetazidine in Patients with angina pectoris having been treated by percutaneous Coronary Intervention (ATPCI) trial – could provide definitive evidence into the clinical benefits of metabolic cardioprotection for patients who have been revascularised, whether for chronic stable angina or acute coronary syndrome.
Short and long-term administration of TMZ has proven to be beneficial in improving clinical parameters and survival in patients with CHF of different aetiology and even in older people.64–70 Indeed, TMZ can improve left ventricular function, exercise capacity and New York Heart Association functional classification, and endothelium-dependent dilation in patients with CHF.50 In addition to these results, in a meta-analysis conducted by Gao et al. TMZ had a significant protective effect for all-cause mortality, cardiovascular events and hospitalisation.71 Further confirming the role metabolic disturbances plays in myocardial ischaemia, TMZ use was associated with improved myocardial perfusion and contractile response in patients with chronically dysfunctional myocardium and ischaemic cardiomyopathy.72
In addition, it has been reported that TMZ has some anti-inflammatory properties because it can attenuate neutrophil activation, thereby protecting post-ischaemic hearts from neutrophil-mediated injury, suggesting a role for this drug in pre- and post-conditioning. TMZ has shown to be cardioprotective when administered before reperfusion. This protection appears to be mediated by activation of p38-mitogen-activated protein kinase and Akt signalling.73
In summary, in patients with IHD, the addition of TMZ has been shown to decrease the amount of angina attacks per week, as well as silent myocardial ischemia episodes; it has reduced the needs for short-acting nitrates and has been shown to improve quality of life. All these results have been accompanied by enhanced exercise tolerance. TMZ has been demonstrated to be effective both in men and women, and in patients with or without diabetes. In patients with CHF, TMZ ameliorates negative left ventricular remodelling, enhances functional capacity, reduces mortality and improves event-free survival. For these reasons as well as its anti-anginal efficacy, TMZ can be used in patients with heart failure and reduced ejection fraction and refractory angina, in addition or as an alternative to conventional medication and is safe for patients with heart failure class IIb, level A.74 Moreover, TMZ has been shown to confer cardioprotection in animal models of ischaemia-reperfusion injury, particularly relevant in patients with stable angina who experience multiple episodes of ischaemia. Generally, TMZ is well-tolerated with minor side effects, mostly comprising gastrointestinal disturbances and headache. However, some patients experience extrapyramidal complications making this drug unsuitable for patients with Parkinson’s disease and similar disorders.75 Precautions should be taken when prescribing to the elderly and those with moderate renal impairment who will need a reduced dose. Although its clinical benefits have been documented since the early 1980s, TMZ still lacks widespread clinical use. It has been classified in European guidelines as a second choice agent for the treatment of chronic ischaemic heart disease, receiving a non-justified class IIb level of recommendation, especially when compared with traditional agents which have been less extensively studied and more largely used.76,77
Ranolazine
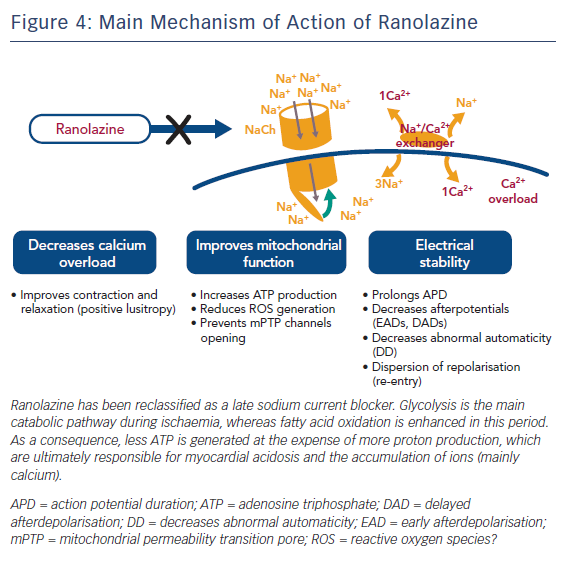
Ranolazine (RNZ) is structurally related to piperazine and is similar to TMZ. It has proved to be effective in patients with chronic stable angina on top of or in combination with traditional antianginal drugs.78 RNZ was shown to display anti-ischaemic properties by promoting glucose oxidation at the expense of fatty acid oxidation (Figure 3).79–81 In addition to this, RNZ induces a reduction in intracellular calcium overload through inhibition of the late sodium channels, with consequent attenuation of oxidative stress (Figure 4).82,83
Randomised clinical trials have demonstrated an improvement in exercise capacity and reduction in angina episodes with ranolazine in patients with chronic stable angina. In patients undergoing elective coronary angioplasty, ranolazine demonstrated a reduction in peri-procedural MI.84 Moreover, in women with evidence of MI, angina and no obstructive coronary stenosis, ranolazine improved quality of life, angina stability and myocardial perfusion reserve index.85 This therapeutic benefit occurs without the haemodynamic effects seen with conventional anti-anginal agents.86 Despite QTc-prolonging action, clinical data have not shown a predisposition to torsades de pointes, and the medication has shown a reasonable safety profile even in those with structural heart disease. RNZ may play a role in the treatment of patients with CHF as its mode of action involves the inhibition of late sodium current and it is able to modulate myocardial metabolism. In an animal model of CHF, RNZ significantly increased left ventricular ejection fraction, peak LV+dP/dt and stroke volume, primarily by optimising cardiac metabolism in the setting of CHF.17 At the cellular level, RNZ influenced hypertrophy, fibrosis and capillary density, as well as the expression for pathological hypertrophy and Ca2+ cycling genes.87
In 2006, RNZ was approved for the relief of angina in patients who remained symptomatic despite taking beta-blockers, calcium channel blockers, or nitrates.88,89 RNZ, even at dosages lower than that currently prescribed, has been shown to confer significant clinical benefit in controlling angina as a monotherapy or an add-on therapy.79–81,90 Short-term use of RNZ therapy has been showed to improve myocardial perfusion and decrease the ischaemic burden, evaluated by single photon emission CT (SPECT).91 The anti-anginal activity of RNZ was tested as a monotherapy in the Monotherapy Assessment of Ranolazine In Stable Angina (MARISA) trial. Compared with placebo, RNZ taken twice daily significantly increased exercise duration, time to onset of angina and time to diagnostic ST-segment depression in 175 patients who were not receiving other anti-anginal medications.81 RNZ was evaluated as combination therapy in the Combination Assessment of Ranolazine In Stable Angina (CARISA) trial. It was assessed in patients with anginal symptoms and reduced exercise capacity despite taking standard doses of atenolol, amlodipine or diltiazem. Exercise duration, time to angina and time to ischaemic ECG changes increased in both RNZ groups versus placebo group (p< 0.01).79 In a post hoc analysis, RNZ 750 and 1,000 mg reduced HbA1c versus placebo; the HbA1c levels appeared to remain unchanged over time during long-term therapy.92,93
In the Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina (TERISA) trial, RNZ reduced the weekly frequency of angina and sublingual nitroglycerin use in subjects with type 2 diabetes, coronary artery disease and chronic stable angina who remained symptomatic despite treatment with up to two anti-anginal agents.94 The therapeutic benefits of RNZ were shown to be greater in those with higher HbA1c values.95
RNZ has proven to be effective not only in patients with coronary artery disease, but also in those with microvascular dysfunction.96 Compared with placebo, patients on RNZ had significantly better Seattle angina questionnaire scores, including physical functioning, angina stability and quality of life. There was a trend towards better mid-ventricular perfusion when using RNZ. Among women with coronary reactivity testing, those with coronary flow reserve (CFR) ≤3.0 had a significantly improved perfusion on RNZ versus placebo compared with women with CFR >3.0.95 This phase 2 study provided the basis to conduct a definitive large clinical trial to evaluate the role of RNZ in microvascular coronary dysfunction.85
Despite original observations, RNZ has been shown to improve non-invasive CFR in women with MI and no obstructive coronary atherosclerosis.97
When evaluated for chronic stable angina, as in the Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST Elevation Acute Coronary Syndromes (MERLIN-TIMI 36) trial, the addition of RNZ to standard treatment was not effective in reducing major cardiovascular events.98 However, RNZ proved to be more effective in reducing recurrent ischaemia in this high-risk population, having particular efficacy in reducing recurrence of ischaemic events in women without increasing the risk of fatal arrhythmias, despite QT prolongation.99–101 In a post hoc analysis, in patients with BNP >80pg/ml, RNZ reduced the primary end point (a composite of cardiovascular death, myocardial infarction and recurrent ischemia).102 As with the CARISA trial, RNZ treatment improved glycaemic control, reducing fasting plasma glucose and HbA1c levels.93 Despite such promising results, in the subset of patients with stable angina that had been incompletely revascularised the Ranolazine in patients with incomplete revascularisation after percutaneous coronary intervention [RIVER-PCI] study), RNZ at the doses of 1,000 mg twice a day failed to ameliorate angina and quality of life and to reduce the composite rate of ischaemia-driven revascularisation or hospitalisation without revascularisation.103,104 In a second analysis of the RIVER-PCI trial, specifically evaluating the role of RNZ on angina frequency and quality of life in relation to diabetes, RNZ ameliorated glycaemic control (HbA1c) at 6 and 12 months compared with placebo. In line with this result, in people with diabetes, angina frequency and quality of life was better at 6 months, but remained unchanged at 1-year follow-up. Better results were observed in patients with poor glycaemic control at enrolment (HbA1c≥58 mmol/mol).105
Larger trials are warranted to find out whether RNZ is capable of improving morbidity and mortality. In summary, RNZ appears to be an effective anti-anginal drug with great potential to fill an unmet need in the management of patients with IHD. The availability of an effective, well-tolerated, and apparently safe, anti-anginal medication such as RNZ is particularly important for patients with angina who are not candidates for revascularisation and for patients with persistent angina. This population appear to have an increased risk of developing cardiac arrhythmias and may benefit from the adjunct of RNZ on top of other medication.83,106–108 In addition to these data, new evidence suggest a possible benefit of using RNZ for CHF due to its ability to modulate myocardial metabolism and its action on late sodium current, which may suggest that this drug may offer additional benefits in patients with ischaemic and non-ischaemic cardiomyopathy due to different aetiology.87,109–115 RNZ has a good safety profile with mild gastrointestinal side-effects (constipation and nausea) and dizziness. Caution should be applied when RNZ is used in patients who already take medication to prolong QT and have liver cirrhosis. However, RNZ-induced QT prolongation has not been associated with torsades de pointes. RNZ may be used as an alternative when beta-blockers are not tolerated in patients with heart failure with reduced ejection fraction and ischaemic heart disease. RNZ can also inhibit late Na(+) current, suggesting a clinical role for this drug in patients with long QT syndrome type 3.116–118
RNZ has been a major success and has gained a class IIa level B recommendation for angina relief in both European and American guidelines on management of stable IHD.1,10
Conclusions
Metabolic agents can be recommended in conjunction with or as an alternative when classic anti-anginal agents are not tolerated or they are contraindicated. Their efficacy has been proven in clinical trials in patients with refractory angina, chronic IHD and in patients exposed to ischaemia-reperfusion injury. Moreover, in patients with heart failure, metabolic agents slow the progression of the disease and improve prognosis. The failing and the ischaemic heart are energy-starved organs dependent on inefficient fatty acid oxidation. Metabolic agents induce a switch in substrate use, rendering the heart more oxygen efficient. Through this and other mechanisms, modulation of cellular energetics has the potential to improve cardiac performance and reduce symptoms in patients with chronic stable angina without relying on alteration of haemodynamics or further neuro-hormonal modulation. As such, agents acting with this approach are likely to complement or substitute, rather than mimic, established therapy and hold a possibility for clinical benefit in a wide range of cardiovascular disorders.
Cardiac metabolic modulators open the way to a better understanding of ischaemic heart disease and its common clinical manifestations, where myocardial ischaemia is no longer considered as a mere imbalance in oxygen and metabolites demand/supply but as an energetic disorder. A better understanding of the mechanisms underlying IHD and chronic stable angina helps in the selection of the most appropriate agents, to properly design clinical trials, to improve symptoms and to improve patient prognosis.