Warfarin is a widely used anticoagulant for the prevention and treatment of thromboembolic disorders. It is well known that there is considerable inter-individual variability in warfarin dose requirements. Furthermore, because warfarin has a narrow therapeutic window, there is a risk of serious sequelae, such as thromboembolism or bleeding, if international normalised ratio (INR) levels fall into the sub- or supra-therapeutic range, respectively. Dosing is highly individualised and is affected by various factors, including age, ethnicity, concomitant drugs used, nutritional status and acute and chronic disease states, among others. Maintenance doses in patients have been observed to range from as little as 1 to >10 mg/day. This leads to significant delays in achieving INR within the therapeutic range, especially when prescribers are inexperienced with warfarin titration.
Genetic polymorphisms are important factors affecting an individual’s dose requirements for warfarin. Genetic variants of the vitamin K epoxide reductase complex subunit 1 (VKORC1) gene, which encodes the target enzyme of warfarin, the cytochrome P450 family 2 subfamily C member 9 (CYP2C9) gene, which is the main enzyme involved in warfarin metabolism and the cytochrome P450 family 4 subfamily F member 2 (CYP4F2) gene, as well as other non-genetic factors, such as age, smoking status, concomitant drugs and co-morbid conditions, account for approximately 50% of the variability in warfarin dose requirements, and up to 60% in Singapore.1,2 Patients who have the VKORC1 –1639 G>A variant are more sensitive to warfarin, and those who carry the decreased function alleles CYP2C9*2 and CYP2C9*3 have reduced dose requirements due to impaired metabolism.
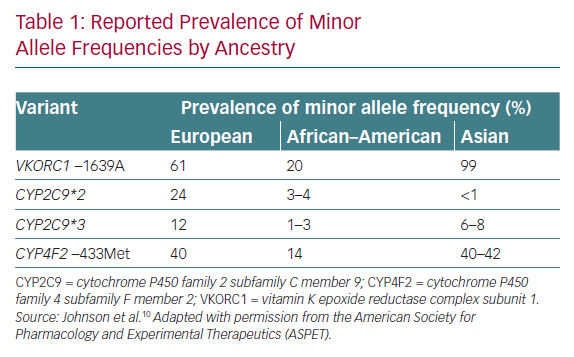
The effects of genotype on warfarin dose are well recognised, as evidenced by drug labels such as those of the Food and Drug Administration and the Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for pharmacogenetics (PGx)-guided warfarin dosing.1 The CPIC guidelines strongly recommend a PGx-guided approach in patients of non-African ancestry, although there is a difference in the spread of genetic variants between Caucasians and Asian populations (Table 1) that makes these recommendations challenging to contextualise.1 In general, Asians are under-represented in validated models used in clinical trials, making it difficult to routinely apply the recommendations in Asia.3
When faced with heterogeneous patient populations in our practice, which includes Asian patients, how should we be tackling these differences and can genotyping help? What is the current evidence for and against warfarin genotyping, and how should it be positioned based on what we know? This article briefly reviews the current evidence surrounding genotype-guided dosing and discusses the role of genotyping in an Asian context, such as Singapore. In addition, we share our experience of implementing genotype-guided warfarin dosing and our opinion on its usefulness in the real-world setting.
Key Trials for Genotype-guided Dosing
Although the latest disease-specific major society guidelines mention the effect of genotype on warfarin dose, they do not recommend routine testing. In 2013, two important studies were published. The first of these studies was the European Pharmacogenetics of Anticoagulation Therapy (EU-PACT) trial, considered a ‘positive’ study, which compared PGx-guided dosing versus fixed dosing in a homogeneous European population (n=445).4 At 12 weeks, the primary outcome of the percentage of time in the therapeutic range (%TTR), as well as the secondary endpoints of INR ≥4 and time to attain stable dosing, were better in the PGx-guided dosing than the comparator arm.4 The second study was the Clarification of Optimal Anticoagulation Through Genetics (COAG) trial, comparatively thought of as the ‘negative’ study, which was performed in an ethnically diverse North American population (n=1,015).5 The COAG trial compared PGx-guided dosing versus a clinical algorithm that took into consideration age, body size, interacting drugs and other factors. The results of COAG showed no difference in time to stable dose, %TTR or a reduction in the number of episodes with out-of-range INR values or bleeding.5 The absence of recommendations regarding the routine use of PGx-guided warfarin dosing is likely due to the conflicting findings from these two clinical trials.
These two large trials, with essentially the same study design but performed in different geographical locations and yielding different results, illustrate the complexities in interpreting studies on PGx-guided dosing. Plausible explanations for the discordant findings lay in the different dosing strategies for the comparator arms, as well as in the ethnic make-up of the study populations. In terms of dosing strategies, the comparator arm in COAG used a clinical algorithm, whereas the EU-PACT trial used an algorithm with fixed doses. The clinical algorithm would be expected to perform better because it accounts for various factors affecting anticoagulation, unlike the fixed-dose approach, which is actually more pragmatic and reflective of actual practice. In terms of ethnic make-up, the COAG trial included a heterogeneous population comprising 30% African–Americans, whereas EU-PACT was performed on a homogeneous European population. African–Americans carry additional variants of CYP2C9*5, CYP2C9*6, CYP2C9*8 and CYP2C9*11, and the lack of inclusion of these variants in COAG was a possible factor in reducing the accuracy of the predicted dose. Because the results from COAG suggested harm in the African–American subgroup, CPIC discourages PGx-guided dosing for African–Americans if testing for the additional variants is not available. In conclusion, the results of these trials indicate that a good understanding of the factors contributing to the accuracy of predicted doses is instrumental in determining how useful a genotype-guided dosing strategy will be.
Genotype-guided Dosing in Singapore
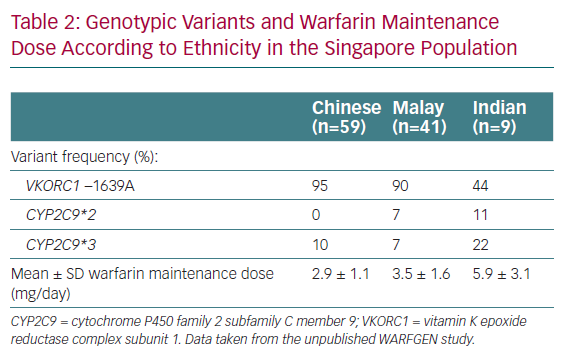
We are fortunate to have data available regarding PGx-guided warfarin dosing in Singapore.6 Singaporeans are heterogeneous in genetic make-up, as seen in the genotype differences observed thus far. There are three main ethnic groups in Singapore, namely Chinese (~74% of the population), Malay (13%) and Indian (9%), with a small proportion of Caucasian and other ethnicities (3%).7 Clear interethnic genotypic differences have been reported for the Singaporean population.8 Specifically, Chinese and Malays have lower warfarin requirements, reflective of the typical ‘Asian’ profile, whereas Indians require higher maintenance doses, more closely resembling the ‘Caucasian’ profile.
Local Singaporean data regarding PGx-guided warfarin dosing appears reassuring. Locally developed dosing algorithms incorporating VKORC1 and CYP2C9 status predicted up to 60.2% and 73.4% of variability in dose requirements among Chinese patients and the population as a whole, respectively.2,9 In 2018, the results of an open-label randomised trial consisting of 322 patients and testing the utility of the algorithm of Tham et al.2 were published.6 In that study, the PGx-guided approach significantly reduced the number of dose titrations within the first 2 weeks (1.77 versus 2.93; p<0.001 for both non-inferiority and superiority) and the number of dose adjustments required over a 90-day follow-up period (4.51 versus 6.06; p=0.001) compared with a traditional dosing approach. Both approaches had similar efficacy (%TTR at 3 months 60.0% versus 57.1%; p=0.29) and comparable rates of bleeding events.6 These results suggest that dose titrations could potentially be reduced by approximately 30%, translating into reductions in hospital length of stay and the number of outpatient appointments.
Determining the Best Algorithm for Genotype-guided Dosing
With numerous algorithms available in the literature, how does one decide on the best algorithm for patients? We aimed to answer this question by performing a correlation study using two different algorithms to predict maintenance dose. All-comers newly initiated on warfarin for indications requiring therapy for a minimum of 3 months and those who had never been on a stable dose of warfarin before were included in the study. Predicted doses were calculated for each patient using two algorithms, namely the validated Gage algorithm used in the COAG trial5 and the locally developed algorithm by Tham et al.2 The predicted doses were then compared to the actual maintenance dose for each patient. The results showed that the mean prediction error, calculated by the mean difference between the predicted and actual stable doses, was 0.4 ± 1.3 mg/day with the Gage algorithm and 0.1 ± 1.3 mg/day with the Tham algorithm (Chang et al., unpublished data, 2015). The doses predicted by the two algorithms had moderately strong correlations with the actual stable dose (R2=0.69 and 0.68 for the Gage and Tham algorithms, respectively). The doses predicted by both algorithms were strongly correlated with each other (R2=0.95). Compared with a cohort of 81 patients who received standard dosing, our PGx-guided dosing arm trended towards achieving a stable dose more quickly (16.0 versus 18.5 days; p=0.49) with a commendable 90-day %TTR of 71.1%. There were no bleeding or thromboembolic events in either group. The mean daily dose of warfarin in patients who achieved a stable dose according to ethnicity was 2.9 ± 1.1 mg/day for Chinese, 3.5 ± 1.6 mg/day for Malays and 5.9 ± 3.1 mg/day for Indians; which mirror our observations in practice and further confirm the ‘heterogeneity’ of our population. In addition, the data confirmed the spread of genotypic variants in Singapore (Table 2). Based on these results, we concluded that either algorithm can be used for PGx-guided dosing, particularly the Gage algorithm, which can be accessed at WarfarinDosing.org.
To summarise local findings, genotyping appears to be safe and efficacious and can reduce the number of dose titrations. Unlike in the COAG study, even though our study population appeared heterogeneous, we managed to demonstrate good anticoagulation control with a %TTR of >70%. This is likely because our local patients did not have rare alleles like CYP2C9*5, CYP2C9*6, CYP2C9*8 and CYP2C9*11, which was the issue with the subgroup in COAG.5
Which Populations Would Benefit from Genotype-guided Dosing?
Below, we discuss which patient populations would likely benefit from genotyping. In terms of positioning, we believe genotyping is best used locally as an enabler to reduce healthcare resources needed for anticoagulation management. Two possible scenarios are discussed below: MI patients with left ventricular (LV) thrombi, in whom warfarin is used exclusively; and cases in which there is the possibility of significant drug–drug interactions with warfarin.
Patient Groups in Whom Warfarin is Used Exclusively
There are insufficient data for the use of direct oral anticoagulants (DOACs) in patients with MI who have LV thrombi, and so warfarin is used exclusively in this patient group. Most of these patients are young males with no significant past medical history and are otherwise fit for discharge after coronary revascularisation. Instead, they remain hospitalised for periods up to 1 week purely for warfarin titration, with no other active medical issues other than receiving subcutaneous enoxaparin and waiting for their INR to rise.
With PGx-guided estimation of the maintenance dose, loading doses can be administered with greater confidence, and patients can be discharged early with enoxaparin to self-administer and a same-week outpatient appointment to return for INR monitoring. This strategy expedites the freeing-up of precious hospital beds and allows patients to return to their family and work commitments sooner. At our institution (Khoo Teck Puat Hospital), approximately eight patients newly start warfarin each month (close to 100 patients per year) for LV thrombus after MI. Bearing in mind Khoo Teck Puat Hospital is the smallest restructured hospital in Singapore by bed size, and adding up the numbers from all other institutions, we estimate that warfarin genotyping may benefit close to 1,000 patients per year in Singapore.
Drug–Drug Interactions with Warfarin
Another scenario in which genotyping could be positioned is when there are significant drug–drug interactions with warfarin. Patients on concomitant antiretroviral, antituberculosis and antiepileptic treatments receive warfarin exclusively because concomitant DOAC use is contraindicated and poorly studied. Warfarin dose fluctuations are even more unpredictable in these patients, with the only solution being to monitor and order blood draws even more frequently than usual.
We suggest that genotyping be used to first derive a predicted maintenance dose in the absence of the drug interaction and then to adjust the predicted dose up or down based on the nature of the interaction. This reduces unpredictability in the initiation phase and reduces the number of titrations, translating to fewer outpatient visits. A reduction in the number of appointments and blood draws would be especially appreciated by the patients, because they are likely to be already laden with multiple specialist appointments for their various medical conditions. Although this scenario is less common than the one described above, as we move towards patient-centred care we believe that the time and effort saved would be significant for each individual patient.
With regard to the specific populations highlighted above, we are further exploring the comparisons in a larger cohort as part of the iRight4Me program at our institution because of the large burden of LV thrombus observed. We look forward to sharing our results and experiences in subsequent publications.
Cost-effectiveness of Genotype-guided Dosing
Concerns about genotyping involve the question of cost-effectiveness; this has not been formally studied in Singapore. However, with advances in technology, the cost of genotyping can now be comparable to that of routine investigations. In Singapore, a patient’s out-of-pocket cost for genotyping is approximately US$75 (SG$100). In comparison, one outpatient-based visit, comprising an Anticoagulation Clinic consult and a blood draw to monitor INR, costs approximately US$50 (SG$70) with opportunity costs of half a day off work or time away from daily commitments. One hospital bed day saved to monitor INR would translate to cost savings of approximately US$850 (SG$1,200) for the institution and US$150 (SG$200) out-of-pocket costs for the patient after implementation of government subsidies. Extrapolating the findings of Syn et al., who found that 30% of dose titrations could be saved with genotyping-guided dosing, warfarin genotyping, which only needs to be performed once in each patient’s life, would more than pay for itself if positioned for the working individual or that caregiver who has to accompany an elderly patient to clinic appointments.6
Conclusion
Based on our experience in Singapore, despite a heterogeneous population the genotype-guided predicted and actual maintenance doses were moderately correlated (R2≈0.7). With the advent of DOACs, most patients will receive DOACs, but a subgroup will still require warfarin for LV thrombi, multimorbidity or renal impairment. It would be acceptable to implement warfarin genotyping as a means of saving costs through avoided appointments and reduced hospital length of stay. Finally, there is a lack of evidence for genotyping in the multimorbid population, and future studies should investigate genotype-guided warfarin dosing in the aforementioned special populations. We look forward to sharing our experience in these populations from the iRight4me program at our institution.